CO is stable , but analogous SiO is not stable . Why ?
Video Solution
Text Solution
CO is a resonance hybrid of the following two strucures :
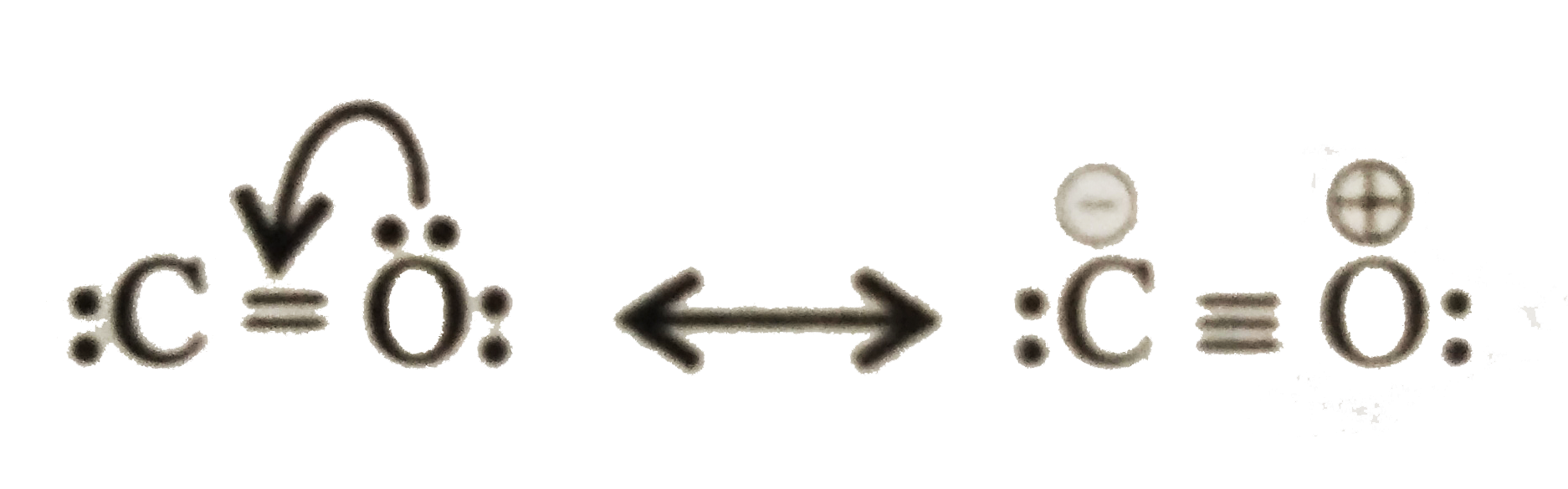
Thus CO contains pπ−pπ multiple bonds. Carbon has a strong tendecy to form pπ−pπ multiple bonds due to its small size and high electrongativeiy. On the other hand Si, due to its large size and less elecrongativty as compared to C , has no tendcy to form pπ−pπ multiple bond. Hence Si does not form SiO molecule andlogouse to CO molecule .
|
Similar Questions
Knowledge Check
Which is not stable
AKHF2BKI3CCH3−CH(OH)2DCl3C−CH(OH)2Which of the following is most stable ?
APh3C+BPh2CH+CPhCH+2DTroplium cation.Statement-1: Co(CO)4 is not stable but [Co(CO)4]− is stable.
Statement-2: [Co(CO)4]− obeys EAN ruleAStatement-1 is True, Statement-2 is True,
Statement-2 is a correct explanation for Statement-1.BStatement-1 is True, Statement-2 is True,
Statement-2 is NOTa correct explanation for Statement-1.CStatement-1 is True, Statement-2 is False.DStatement-1 is False, Statement-2 is True.
(a) How does bond energy vary from N+2 and N−2 and why ?
(b) On the basis of the molecular orbital theory, what is the similarly between :
(i)F2,O2−2 (ii)CO,N2,NO+.
(c ) Like CO why its analong SiO is not so stable ?
View SolutionWhich one is more stable and why?
View SolutionWhy lithium carbonate is not stable to heat?
View SolutionPbCl2 is more stable than PbCl4 . Why ?
View Solution+5 oxiation state of bismuth is less stable then +3. Why?
View Solution
