Out of CS2 and OCS which have higher dipole moment and why?
Video Solution
Text Solution
The dipole moment of (O=C=S) is higher.
CS2(S=C=S) and OCS(O=C=S) both are linear molecular but bond moment in CS2 cancel out so that the net dipole moment is zero. But in OCS, bond moment of (C=O) is not equal to (C=S). Hence, it has a net dipole moment.
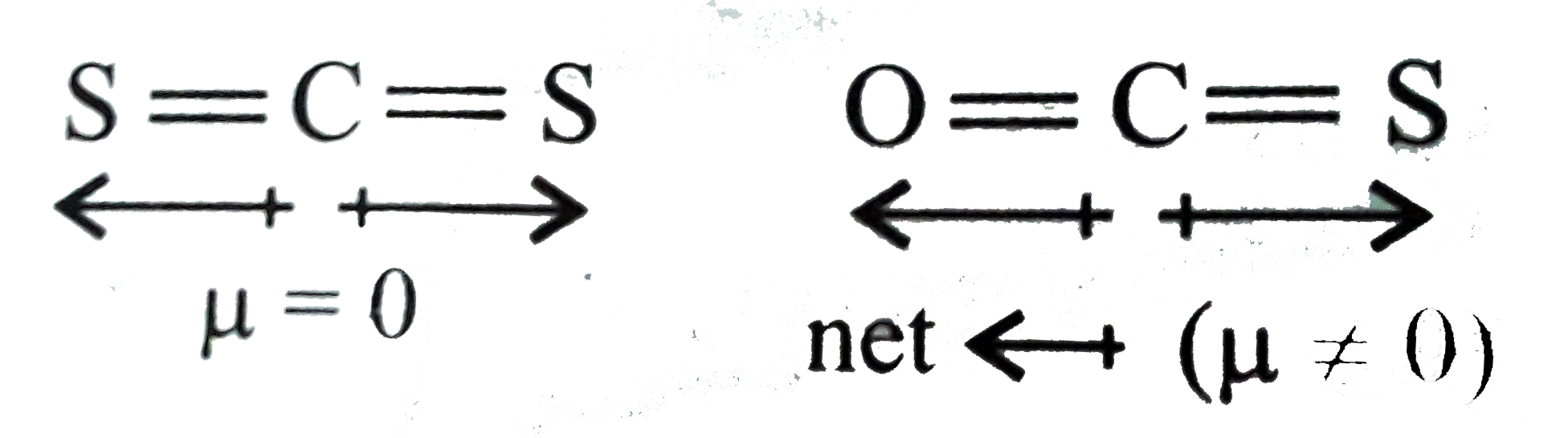
|
Similar Questions
Knowledge Check
Which of the following have dipole moment ?
AnitrobenzeneBp-chloronitrobenzeneCm-dichlorobenzeneDo-dichlorobenzeneThe molecule which have zero dipole moments is .
ACH2CI2BBF3CNF3DCIO2Out of the following which has highest dipole moment?
A2,2-dimethyl propaneBtrans−2- penteneCcis −3−hexenD2,2,3,3− tertamethyl butane
Out of CS2 and OCS which have higher dipole moment and why?
View SolutionOut of but-1-yne or but-1-ene which has higher dipole moment?
View SolutionWhich compound have maximum dipole moment ?
View Solutionwhich of the following have zero dipole moment?
View SolutionThe molecules having dipole moment are :
View Solution
