Write the structures of the following hydrates which contains ionic, covalent, coordinates and H−bonds.
a. CuSO4.5H2O. , b.ZnSO4.5H2O
c. FeSO4.7H2O.
Video Solution
Text Solution
All these hydrates are complex molecules.
a. It is called blue vitriol.
Coordination number of Cu2+=4.So, 4H2O molecules are coordinated with central Cu2+ ion.
It is represented as [Cu(OH2)4]2+SO2−4.H2O.
Four H2O molecules are linked to Cu2+ ion by coordinate bonds and one H2O molecule is linked by H-bonds between a coordinated H2O molecule and SO2−4 ion.
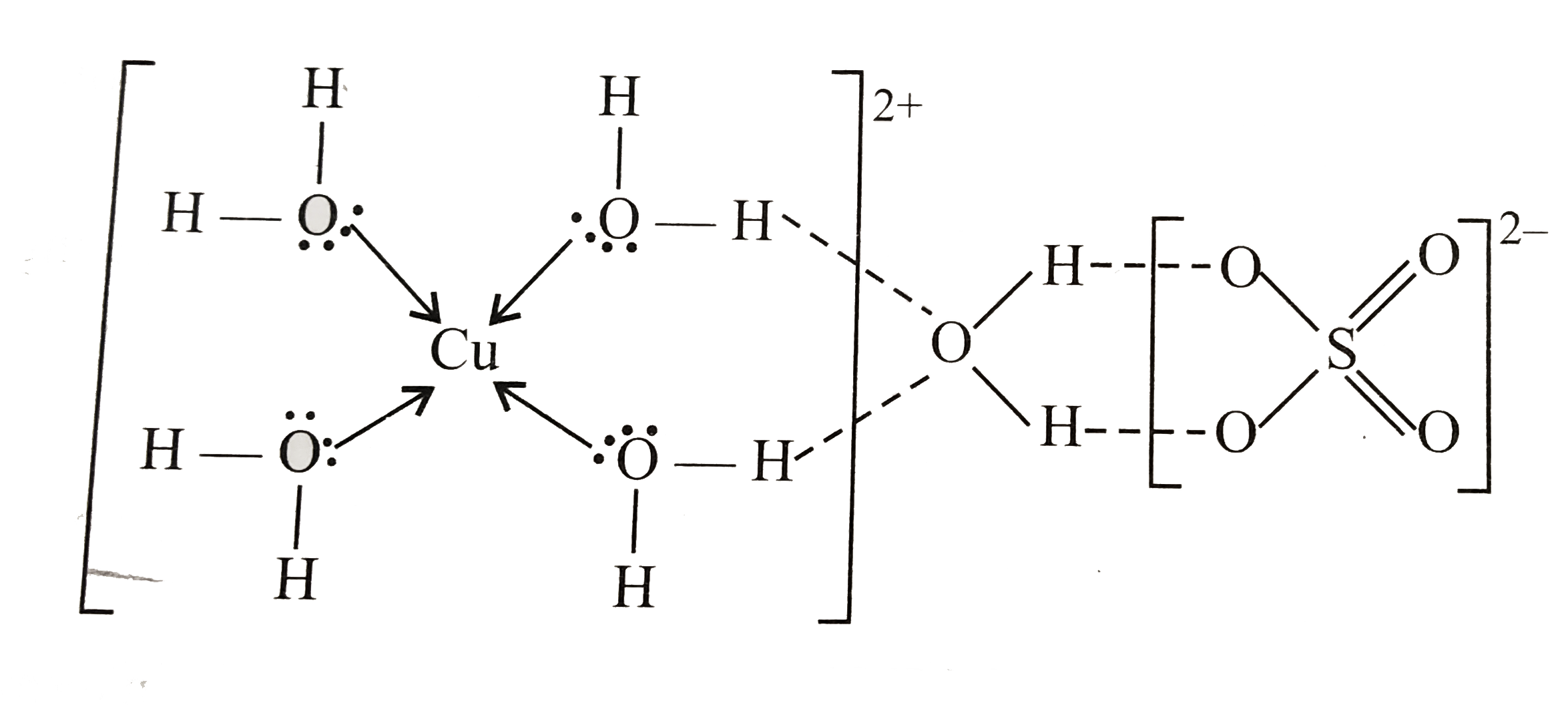
b. ZnSO4.5H2O. It is called white vitriol. Its structure is similar to blue vitriol since coordination number of Zn2+=4.
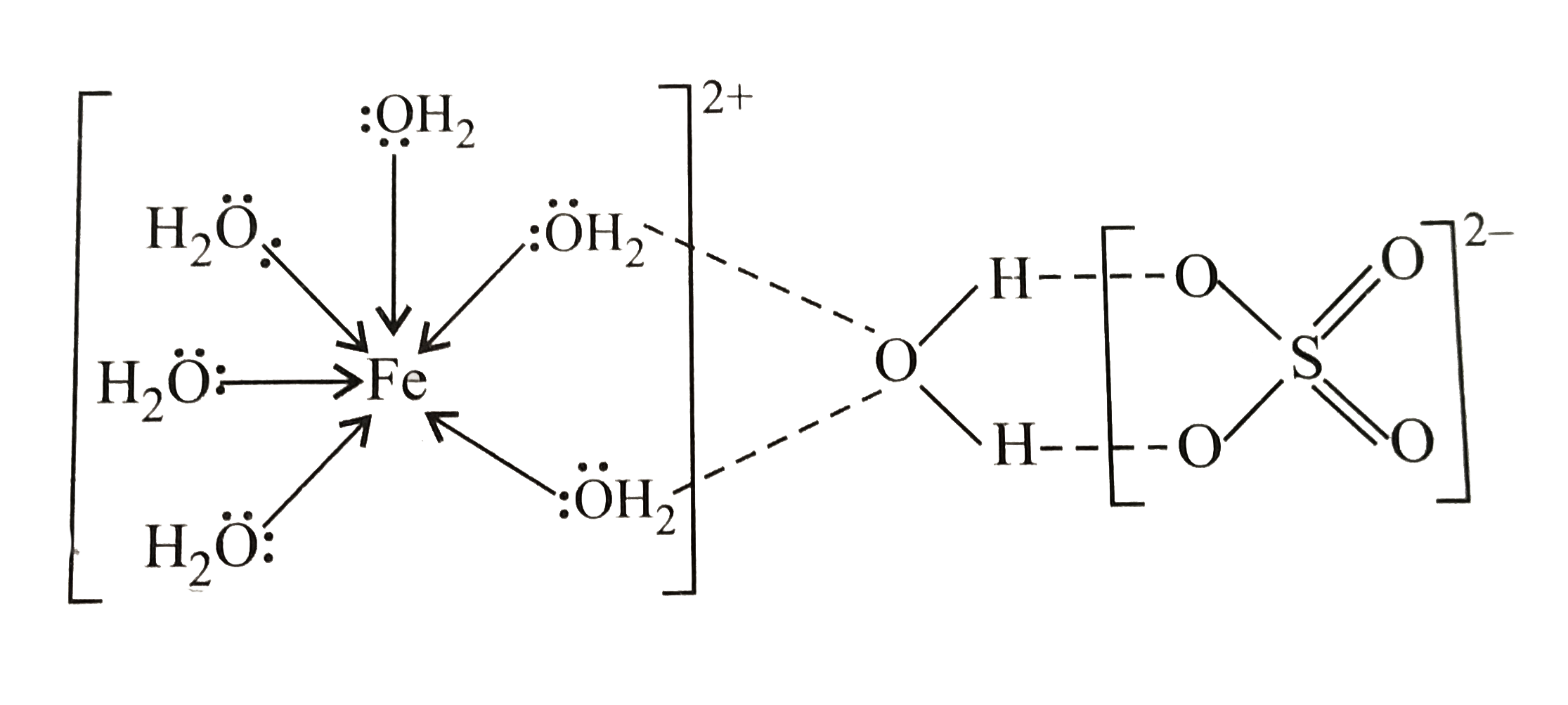
c. FeSO4.7H2O. It is called green vitriol. The coordination number of Fe2+=6. Therefore, 6H2O molecules are linked to Fe2+ ion by coordinate bonds and one H2O molecule is linked by H-bonds between a coordinated H2O molecule and SO2−4 ion.
[Fe(OH2)6]2+SO2−4.H2O.
|
Similar Questions
Knowledge Check
In solid CuSO4.5H2O
ACu2+ ion is coordinate bonded to four water moleculesBOne H2O molecule is H− bondedCIt has four types of bonds, Ionic, covalent, coordinate and H- bondDFive molecules of H2O are coordinate bonded to Cu2+In solid CuSO4.5H2O
AFive molecules of H2O are coordinate bonded to Cu2+BCu2+ ion is coordinate bonded to four water moleculesCOne H2O molecule is H-bondedDIt has four type of bonds : Ionic, covalent, coordinate and H-bondCuSo4.5H2O is a
Asolution of a solid in a liquid.Bsolution of liquid in a solidCsalt only and cannot be called a solutionDcoordination compound of copper with water molecules as the ligands.
Out of the following, select the compounds containing ionic, covalent and coordinate bonds.
CaCl2,C2H6,MgO,HCI,⊕NH4,O3
View SolutionCuSO4⋅5H2O belongs to
View SolutionBonds presents in CuSO4.5H2O is
View SolutionCoordination number of Cu2+ in CuSO4.5H2O is .
View SolutionNumber of water molecules acting as ligands in
CuSO4.5H2O,ZnSO4.5H2O,FeSO4.7H2O respectively are .
View Solution

