Arrange alkaline earth metal flouides in order of increasing solubility in water.
Video Solution
Text Solution
BeF2<MgF2<CaF2<SrF2<BaF2
|
Similar Questions
Knowledge Check
The correct order of increasing solubility in water is:
AKF<NaF<LiFBNaHCO3<KHCO3<RbHCO3CK2CO3<Na2CO3<Li2CO3DLiNO3<NaNO3<KNO3The correct order of increasing solubility in water is:
AKF<NaF<LiFBNaHCO3<KHCO3<RbHCO3CK2CO3<Na2CO3<Li2CO3DLiNO3<NaNO3<KNO3The correct order of increasing solubility in water is:
AKF<NaF<LiFBNaHCO3<KHCO3<RbHCO3CK2CO3<Na2CO3<Li2CO3DLiNO3<NaNO3
Which of the following alkaline earth metal hydroxides is the least soluble in water?
View SolutionWhich of the following alkaline earth metal hydroxides is the least soluble in water?
View SolutionWhich of the following alkaline earth metal sulphates has highest solubility in water?
View SolutionWhich of the following alkaline earth metal sulphate is least soluble in water:-
View SolutionWhen the compounds below are arranged in order of increasing solubility in water, which order is correct?
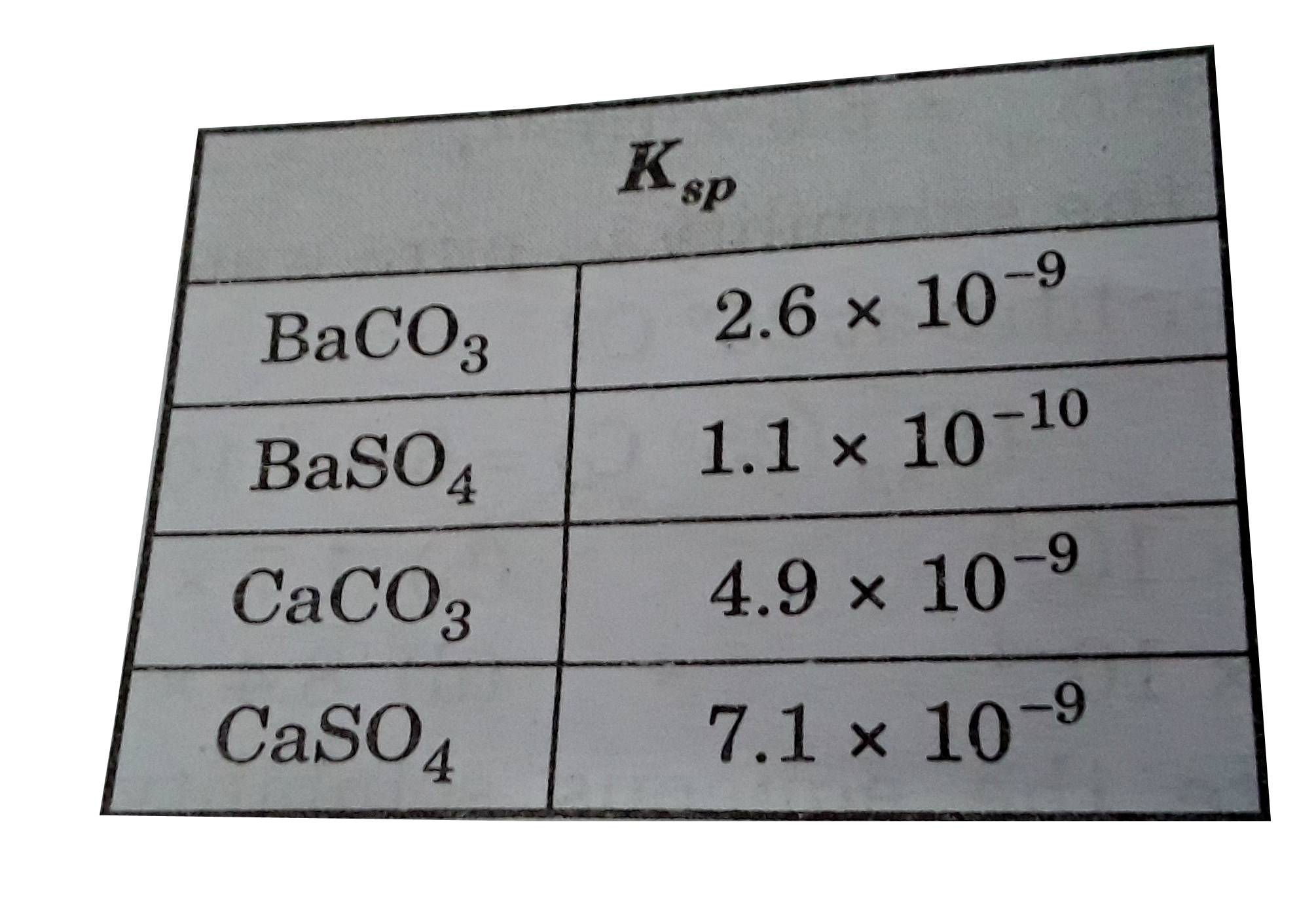
View Solution
