Describe the shapes of BF3 and BHө4. Assign the hybridisation of boron in these species.
Video Solution
Text Solution
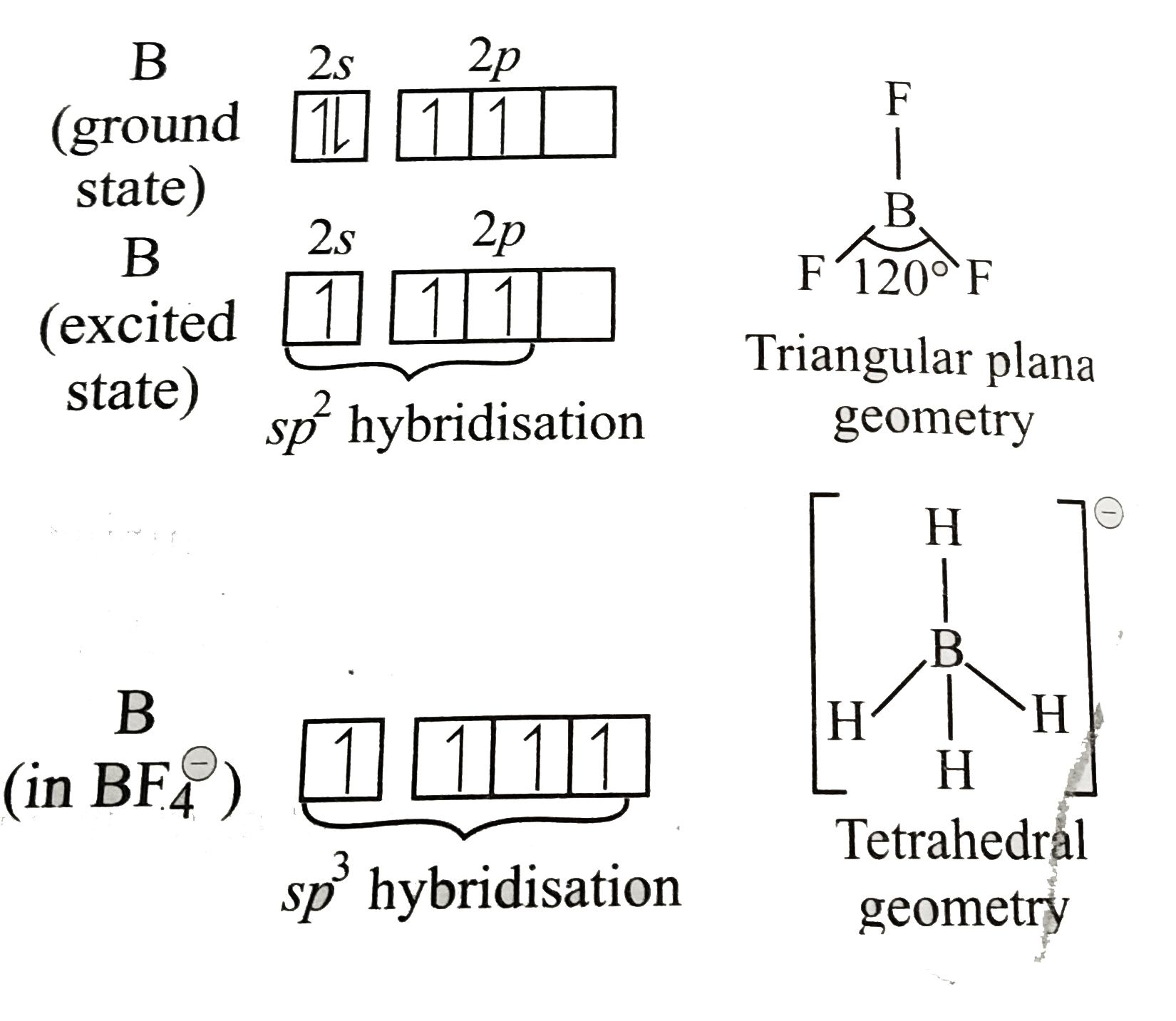
In BF3,B is sp2 hybridised, hence, BF3 has a planar geometry.
In [BH4],B is sp3 hybridised, hence, [BH4]ө has a tetrahedral geometry or shape.
|
Similar Questions
Knowledge Check
The state of hybridisation of Boron in BCl3 is
Asp3Bsp2CspDsp3d2The shape of BF3 species is
ATetrahedralBSquare planarCTrigonal planarDLinearPredict the correct shape of BH−4
APyramidal, sp3Boctahedral, sp3d2Ctetrahedral, sp3Dnone of the above
Write the state of hybridisation of boron in BF3.
View SolutionWrite the state of hybridisation of boron in BF3.
View SolutionIf BF3 react with F−then how will hybridization of Boron change ?
View SolutionDraw the structure of boric acid showing hydrogen bonding. Which species is present in water ? What is the hybridisation of boron in this species ?
View SolutionPredict the correct shape of BH−4
View Solution

