Native silver metel froms a water solube, complesx with a dilute aqueous wsolution of NaCN in the presence of
Nitrogen
Oxygen
Carbon dioxide
Argon
The correct Answer is:B
4Mg+O2+8NaCN+2H2O→4Na[Ag(CN)2]+4NaOH.
Topper's Solved these Questions
- GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTSBOOK - CENGAGE CHEMISTRYCHAPTER - GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTSEXERCISE - Exercise (Assertion-Reasoning)
18 Videos
- GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTSBOOK - CENGAGE CHEMISTRYCHAPTER - GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTSEXERCISE - Exercise (Integer)
27 Videos
- GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTSBOOK - CENGAGE CHEMISTRYCHAPTER - GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTSEXERCISE - Exercise (Multiple Correct)
43 Videos
- ELECTROCHEMISTRYBOOK - CENGAGE CHEMISTRYCHAPTER - ELECTROCHEMISTRYEXERCISE - Archieves Subjective
35 Videos
- GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTSBOOK - CENGAGE CHEMISTRYCHAPTER - GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTSEXERCISE - Exercises Archives (Linked Comprehension)
1 Video
Similar Questions
Assertion: All minerals are ore.
Reason: Ores are minerals from which metal can be extracted conveniently and economically.
View SolutionBauxite ore is concentrated by
View SolutionThe anode mud obtained during electrorefining of copper may contain.
View SolutionA current of 3.7 A is passed for 6hrs. Between Ni electrodes in 0.5 L of 2 M solution of Ni(NO3)2. What will be the molarity of solutionn at the end of electrolysis?
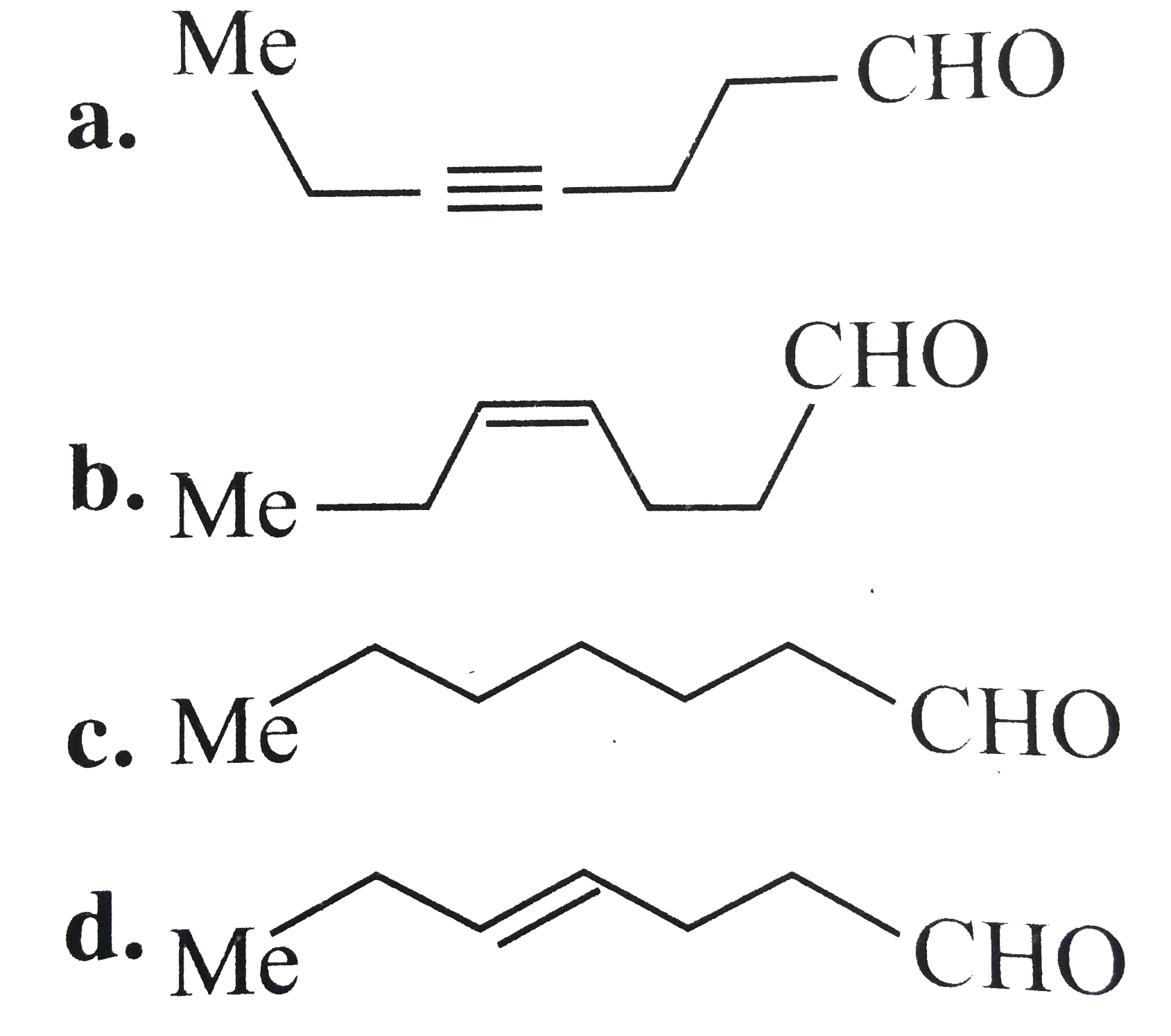
View SolutionIn the following reaction sequence, products I, J, and L are formed. K represents a reagent.
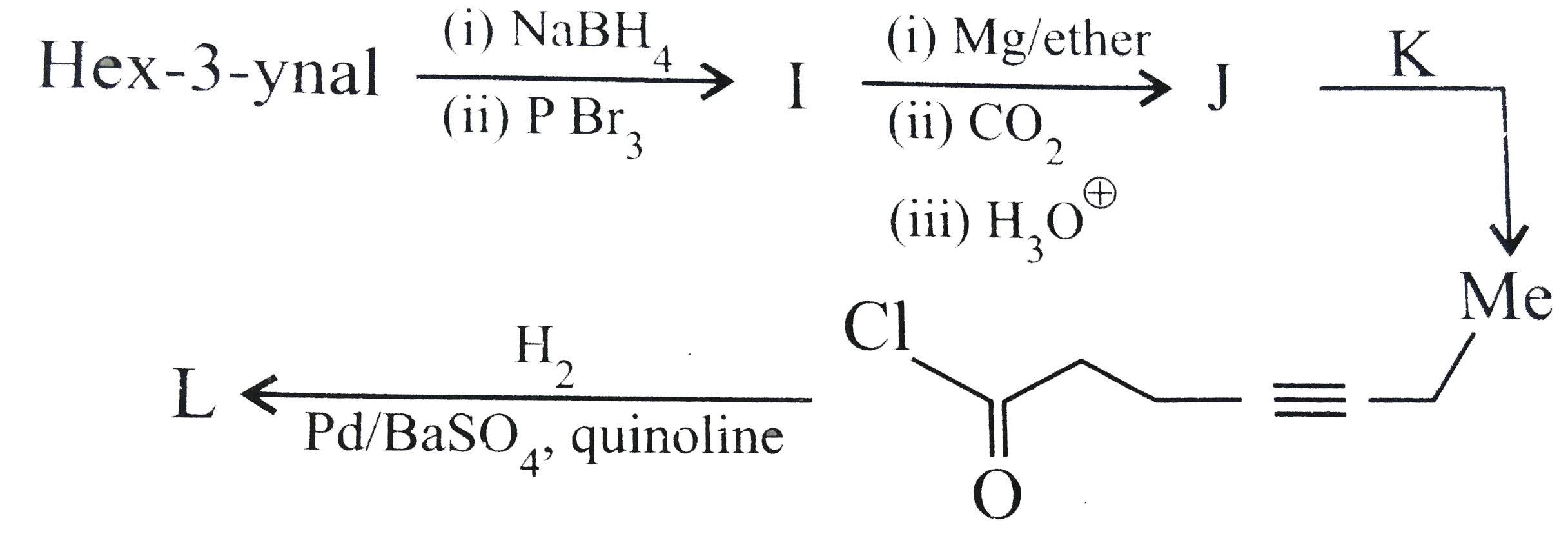
The structures of product I is :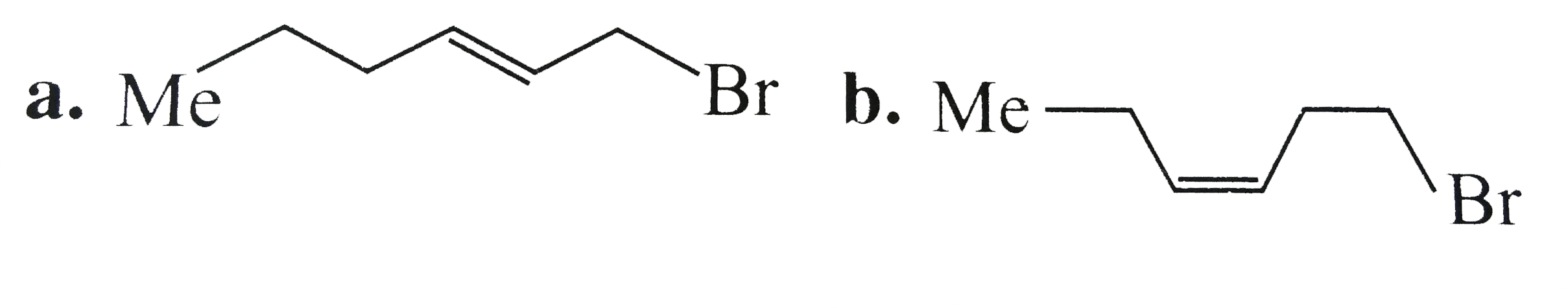
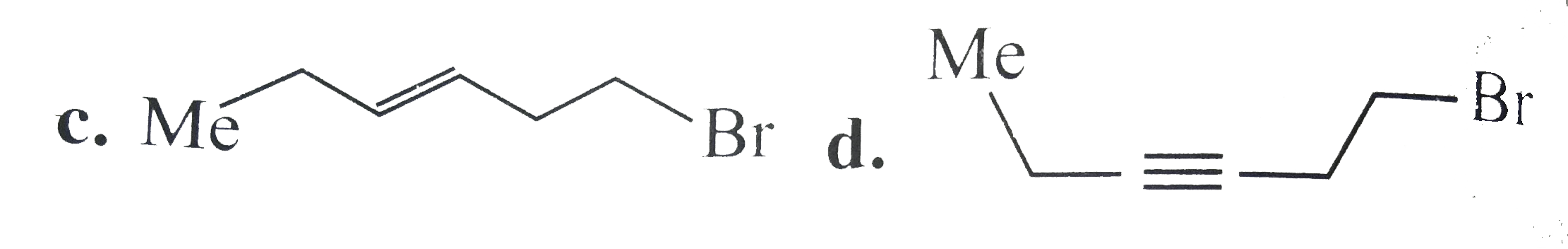
The structures of compounds J and K, respectively, are :
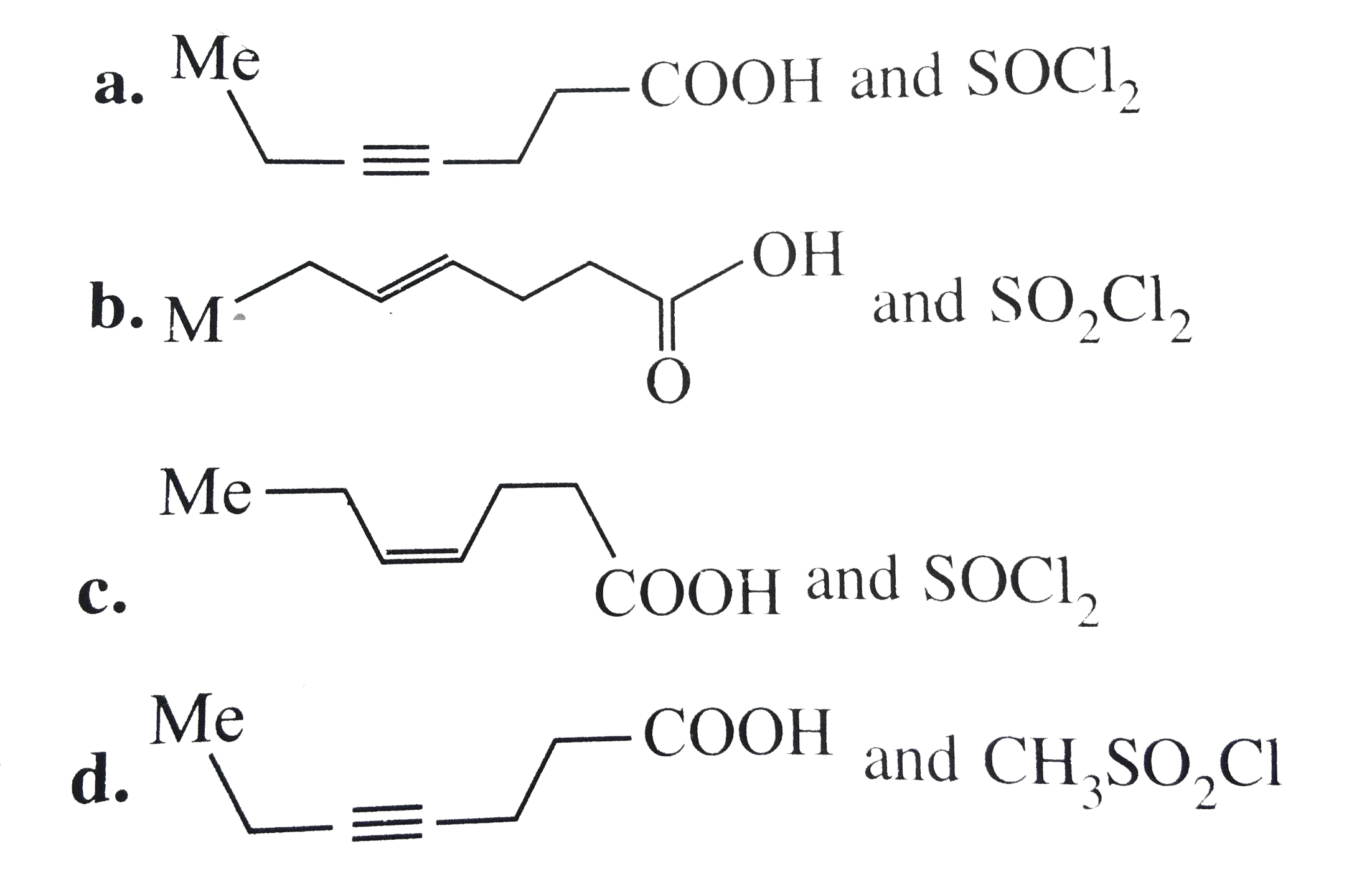
The structure of product L is :
View SolutionNative silver metel froms a water solube, complesx with a dilute aqueous wsolution of NaCN in the presence of
View SolutionNative silver metel froms a water solube, complesx with a dilute aqueous wsolution of NaCN in the presence of
View SolutionNative silver metel froms a water solube, complesx with a dilute aqueous wsolution of NaCN in the presence of
View SolutionSilver ore dissolve in the dilute solution of NaCN in the presence of air to form:
View SolutionNative silver metal forms a water soluble complex with a dilute solution of NaCN in the presence of
View SolutionNative silver metal forms a water soluble complex with a dilute solution of NaCN in the presence of :
View SolutionNative silver metal forms a water soluble complex with a dilute aqueous solution of NaCl in the presence of
View SolutionNative silver metal forms a water soluble complex with a dilute aqueous solution of NaCN in the presence of
View SolutionIn whose presence, the simple silver metal forms water-soluble clusters, with dilute aqueous solutions of NaCN.
View SolutionNative silver metal forms a water soluble complex with a dilute aqueous solution of NaCN in the presence of
View SolutionNative silver metal forms a water soluble complex with a dilute a s wolution of NaCN in the presence of
View Solution

