Which is more basic and why ? NF3 or NH3.
Video Solution
Text Solution
NH3 is more basic than NF3.
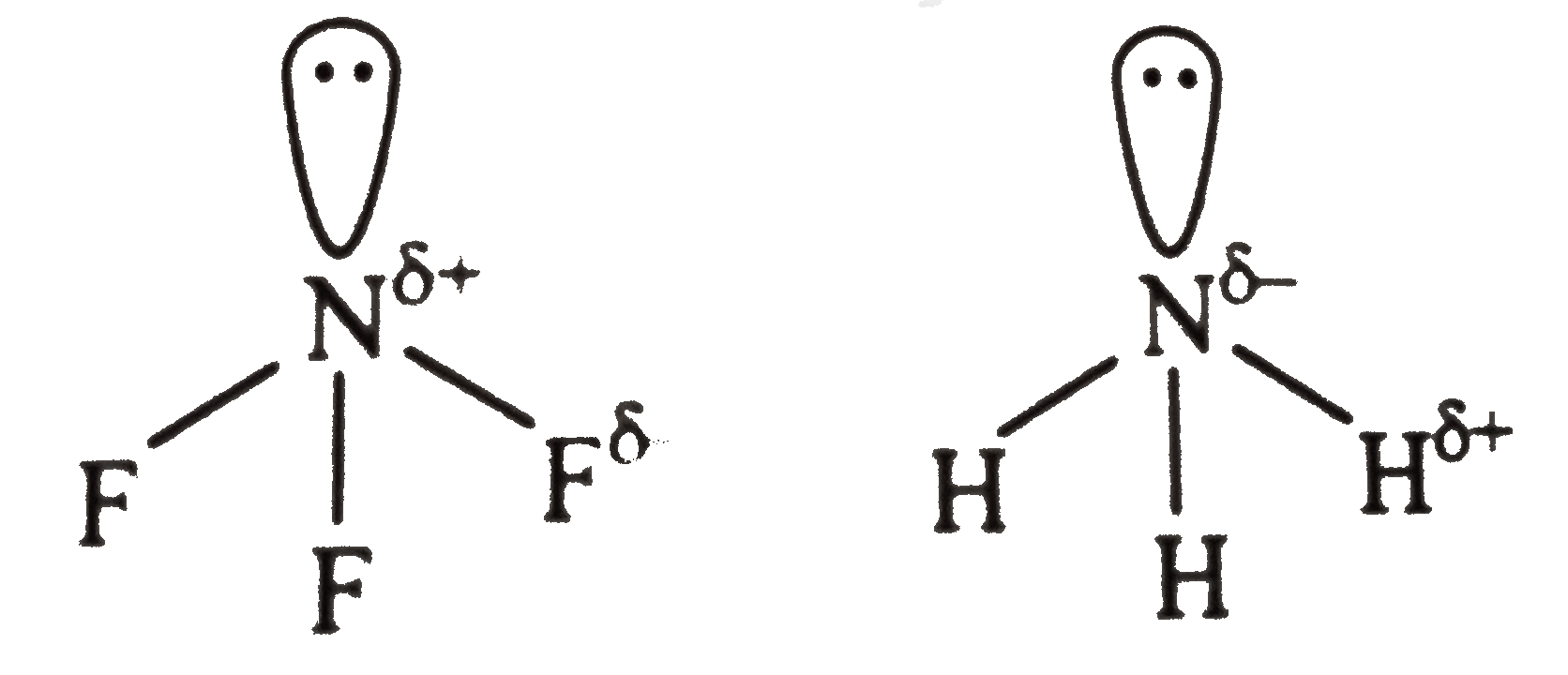
In NF3,N is less electronegative than F, hence bond pair shifts more towards F, thereby δ positive charge density on N increases, hence tendency to donate electron decreases. In NH3,N is more electronegative than H, hence bond pair shifts towards N in N−H, thereby negative charge density on N increases, hence tendency to donate electrons increases. Hence NH3 is more basic as compared to NF3.
|
Similar Questions
Which of the two is more basic and why? CH3NH2 or NH3.
View SolutionWhich is more basic PhNH2 or Ph2NH ?
View SolutionWhich is more basic La(OH)3 or Lu(OH)3 ? Why
View SolutionWhich is more basic La(OH)3 or Lu(OH)3 ? Why ?
View SolutionWhich of the following is more basic/acidic and why?
0.01MHNO3 and 0.001 M HCI
View SolutionPH3 is less basic than NH3.
View SolutionWhich oxide is more basic, MgO or BaO? Why?
View SolutionWhy is aniline less basic than NH3 ?
View SolutionWhich of the following is more stable complex and why? [CO(NH3)6]3+and[CO(en)3]3+
View SolutionWhich of the following is more stable complex and why ?
[Co(NH3)6]3+ and [Co(en)3]3+
View Solution
