What is the total number of diprotic acid among the following ?
H3PO4,H2SO4,H3,PO3,H2CO3,H2S2O7,H3BO3,H3PO2,H2CrO4,H2SO3.
Video Solution
Text Solution
The correct Answer is:(6)
The total number of diprotic acids among the following are :
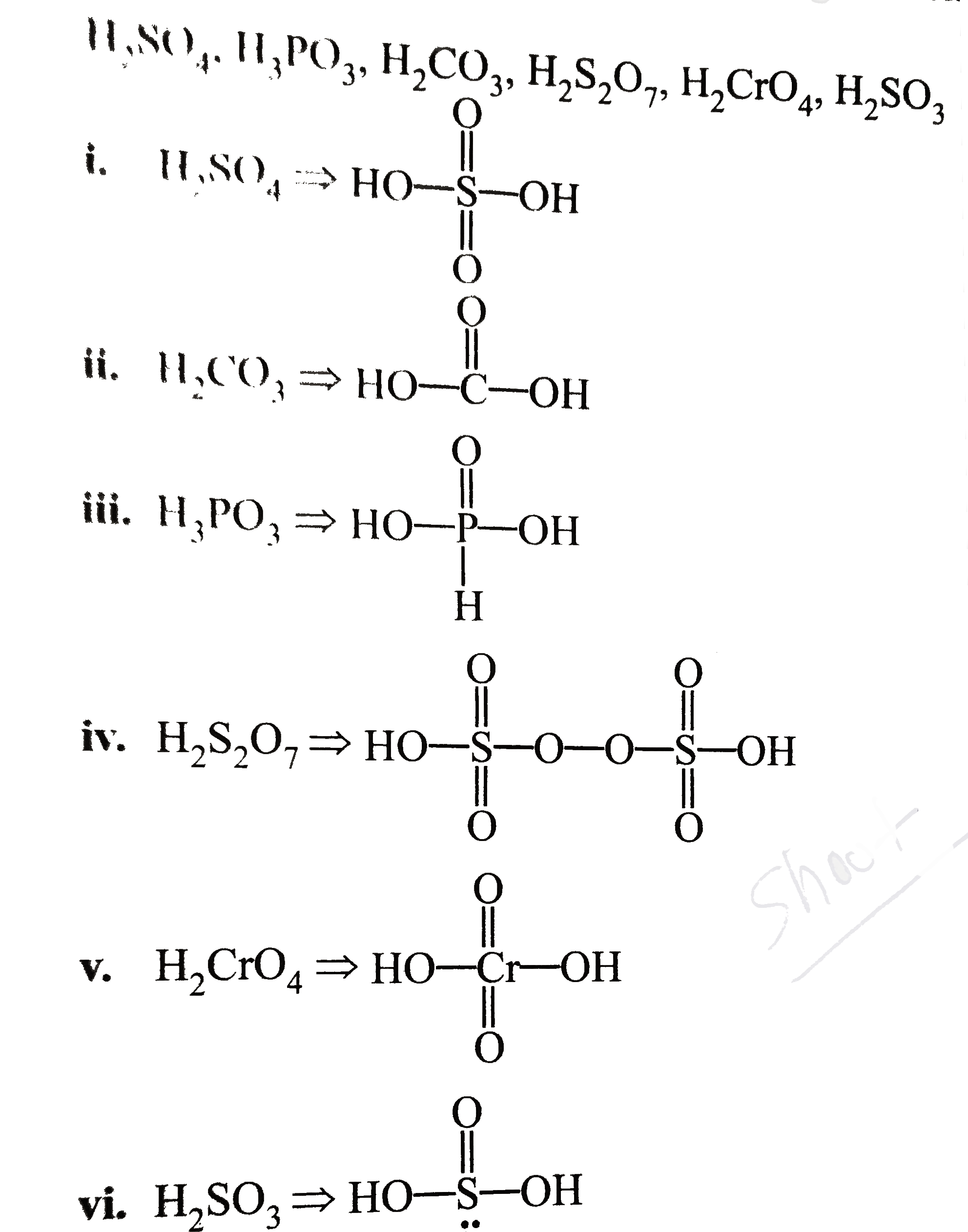 .
.
|
Similar Questions
Knowledge Check
The total number of diprotic acids among the following is
H_3PO_4, H_2SO_4, H_3PO_3, H_2CO_3, H_2S_2O_7,H_3BO_3, H_3PO_2, H_2CrO_4, H_2SO_3A6B7C8D9There is a little difference in acid strength in the series H3PO4,H3PO3 and H3PO2 because
APhosphorus in theses acids exists in defferent oxidation statesBNumber of unprotonated oxygen atoms responsible for increase of acidity due to inductive effect remains the sameCPhosphorus is not a highly electronegative elementDPhosphorus oxides are less basic
The total number of diprotic acids among the following is
H3PO4,H2SO4,H3PO3
H2CO3,H2S2O7,H3BO3
H3PO2,H2CrO4,H2SO3
View SolutionThe total number of diprotic acids among the following is
H3PO4,H2SO4,H3PO3
H2CO3,H2S2O7,H3BO3
H3PO2,H2CrO4,H2SO3
View SolutionThe total number of diprotic acids among the following is
H3PO4,H2SO4,H3PO3
H2CO3,H2S2O7,H3BO3
H3PO2,H2CrO4,H2SO3
View SolutionFind the number of dibasic oxyacids in the following:
H2CO3,H2SO3,H3PO2,H3PO3,H3BO3
Dibasic oxo-acids are H2CO3,H2SO3andH3PO3.
View SolutionThe number of compounds among the following which are monobasic in nature is __________
H3PO3,H3PO2,HClO4,HNO2,H2CO3
View SolutionHow many of these acids are monobasic here?
H3PO2,H3PO3,H2CO3,H2SO4,H2S2O4,H2CrO4,H3BO3,HNO2
View Solution

