Sulphuric acid has high boiling point and viscosity. Why ?
Video Solution
|
Answer
Step by step video & image solution for Sulphuric acid has high boiling point and viscosity. Why ? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
Water has high boiling point because
AIt has high molecules massBIt has high lattice energyCIt is a weak acidDMolecules are associated by hydrogen bondingThe high boiling point and viscosity of H2SO4 is due
Ahydrogen bondingBcovalent bondingCionic bondingDvander Waal's forcesWhich compounds have high boiling point in comparision to
 A
A B
B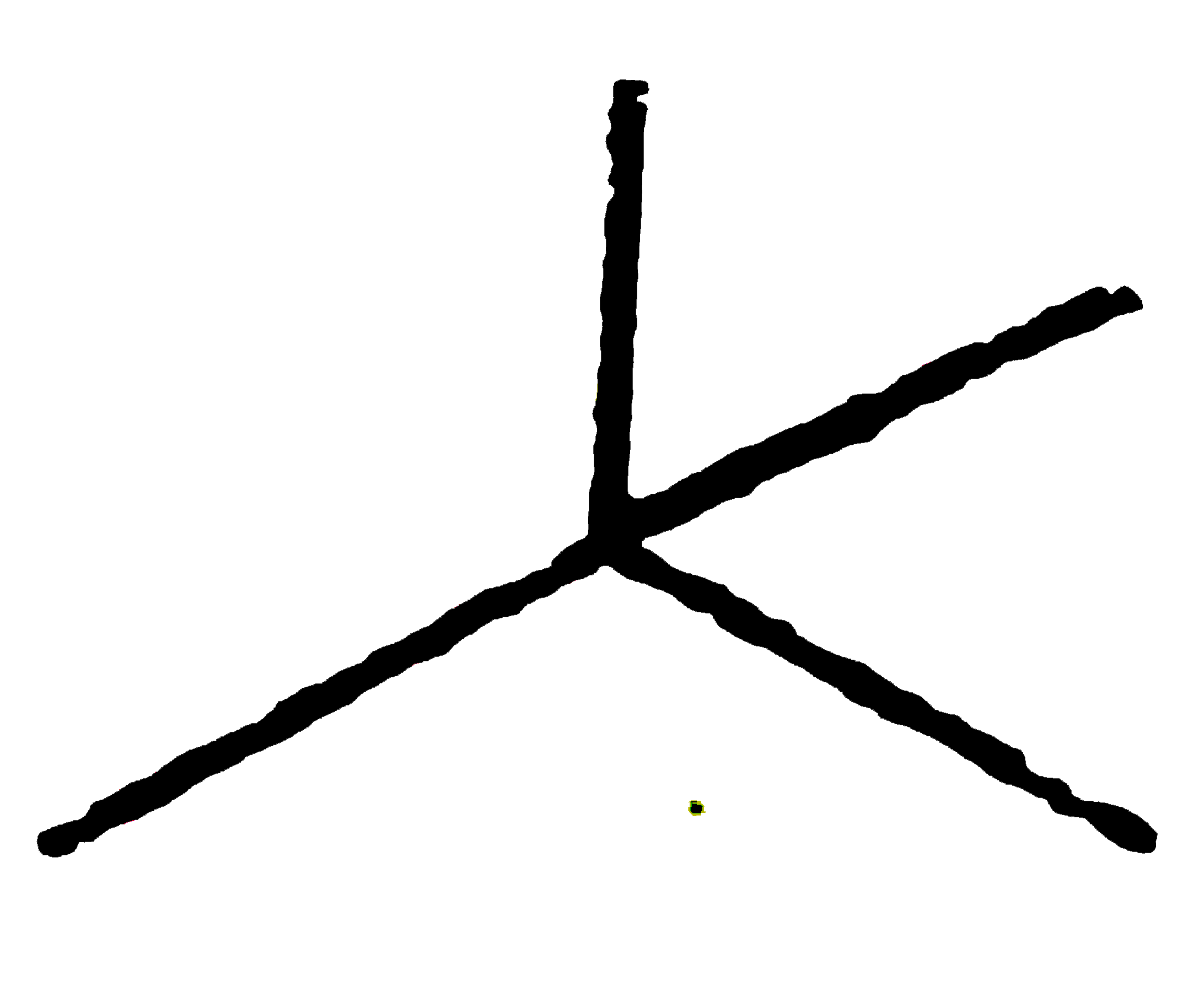 C
C D
D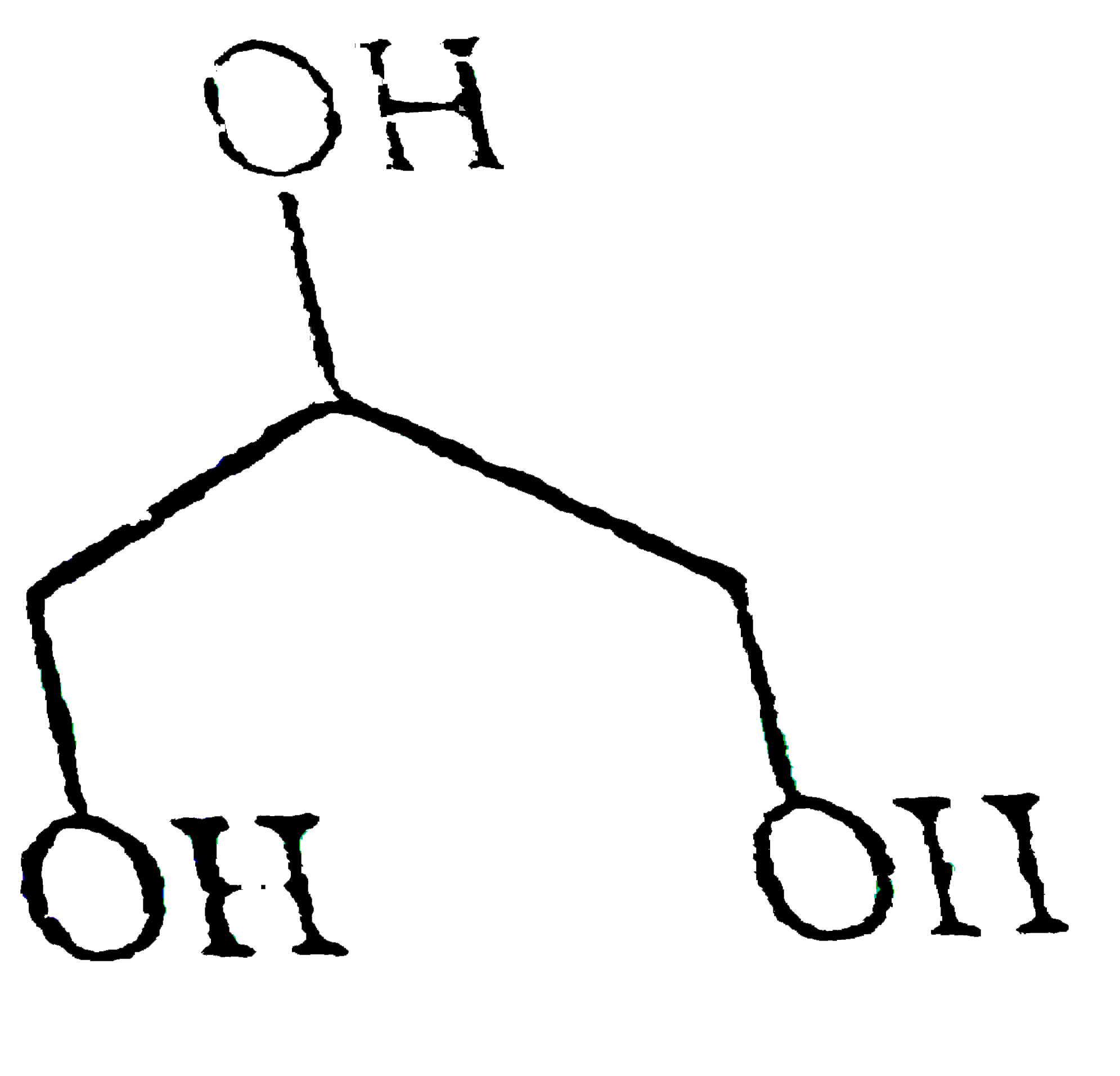
Why do carboxylic acids have higher boiling points than alcohol aof comparable molecular mass?
View SolutionWhich of the two : KClorCl2 has higher boiling point and why?
View SolutionWhy does water have high boiling point?
View SolutionWhich one has high boiling point and why?
Ethyl alcohol or dimethyl ether.
View SolutionHigh boiling point of water is due to :
View Solution
