What happens when :
(a) Ozone is passed through acidified stannous chloride solution.
(b) Potassium iodide is heated with conc.H2SO4.
(c) Sulphur is boiled with caustic soda solution.
(d) Ozone reacts with potassium ferrocyanide solution.
( e) Ozone is treated with ethylene.
(f) Sulphur dioxide is bubbled through aqueous solution of copper sulphate in presence of potassium thiocyanate.
(g) Sulphur dioxide gas, water vapour and air are passsed over heated sodium chloride.
(h) Sulphuric acid is treated with PCl5.
(a) 3SnCl2+6HCl+O3→3SnCl4+3H2O
(b) 2KI+H2SO4→K2SO4+2HI
2HI+H2SO4→I2+SO2+2H2O
(c) 4S+6NaOH→Na2S2O3+2Na2S+3H2OExcess sulphur−−−−−−−−→Na2S5
(d) 2K4Fe(CN)6+O3+H2O→2K3Fe(CN)6+2KOH+O2
( e) 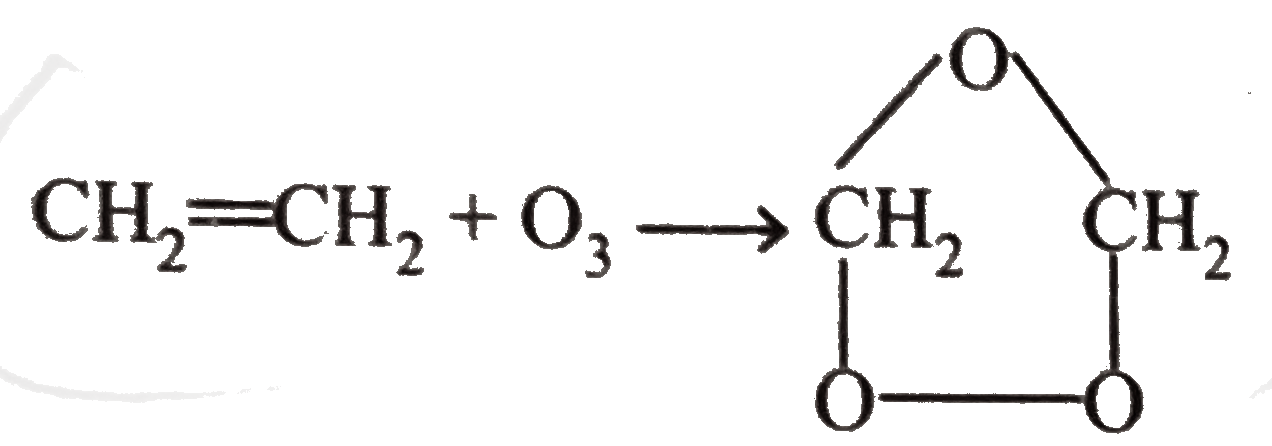
(f) 2CuSO4+SO2+2KCNS+2H2O→2CuCNS+K2SO4+2H2SO4
(g) 2SO2+O2+2H2O→2H2SO4
2NaCl+H2SO4→Na2SO4+2HCl
(h) H2SO4+2PCl5→SO2Cl2+2POCl3+2HCl.
Similar Questions
What happens when SO2 gas is bubbled through an aqueous solution of copper sulphate in the presence of potassium thiocyanate?
View SolutionWhat happens when sulphur dioxide is passed through an aqueous solution of Fe(III) salt ?
View SolutionWhat happens when sulphur dioxide is passed through an aqueous solution of Fe(III) salt?
View SolutionWhat happens when sulphur dioxide is passed through an aqueous solution of Fe(III) salt?
View SolutionWhat happens when sulphur dioxide is passed through an aqueous solution of Fe(III) salt?
View SolutionWhat happens when hydrogen sulphide is bubbled through an aqueous solution of sulphur dioxide.
View SolutionWhat happens, when sulphur dioxide gas is passed through an aqueous solution of a Fe(III) salt
View SolutionWhat happens when sulphur dioxide gas is passed through an aqueous solution of Fe(III) salt ?
View SolutionWhat happens when sulphur dioxide gas is passed through an aqueous solution of Fe(III) salt ?
View SolutionWhat happens when?
(a) Sulphur is boiled with caustic soda.
(b) Sulphuric acid is treated with PCl5
(c) Sulphur dioxide gas, water vapour and air are passed over heated sodium chloride.
View Solution

