The number of unpaired electrons in the valence shell of the members of oxygen family is ____.
Video Solution
Text Solution
The correct Answer is:(2)
Valence shell electronic configuration of members of oxygen family is ns2np4.
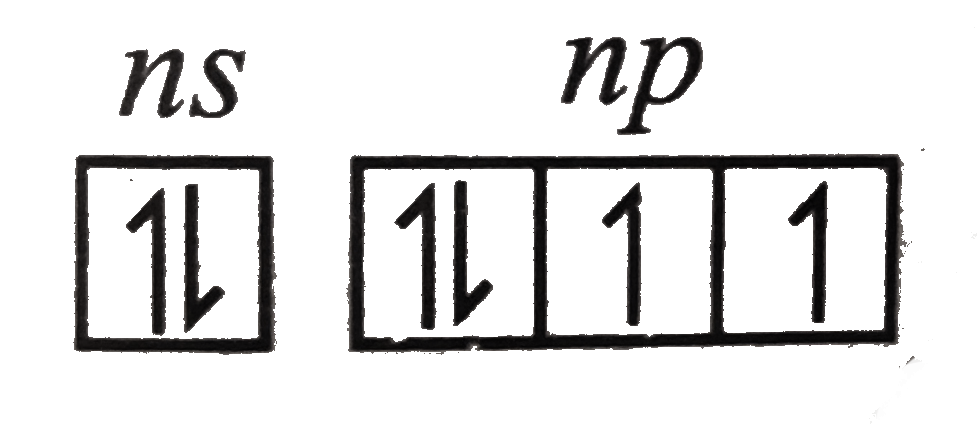
i.e. two electrons are unpaired.
|
Similar Questions
Knowledge Check
The number of electrons in the valence shell of calcium is
A6B8C2D4The number of electrons in the valence shell of calcium is
A6B8C2D4The number of electrons in the valence shell of calcium is:
A2B4C6D8
The number of electrons in the valence shell of a semiconductor is
View SolutionThe number of electrons in the valence shell of nitrogen in an amine is
View SolutionThe number of electrons in the valence shell of sulphur in SF6 is :
View SolutionThe number of electrons in the valence shell of sulphur in SF6 is :
View SolutionThe number of electrons in the valence shell of sulphur in SF6 is
View Solution

