CIF2 exists but FCl3 does not. Why?
Video Solution
Text Solution
As Cl has vacant d-orbital in its valence shell Cl can expand its coordination number.
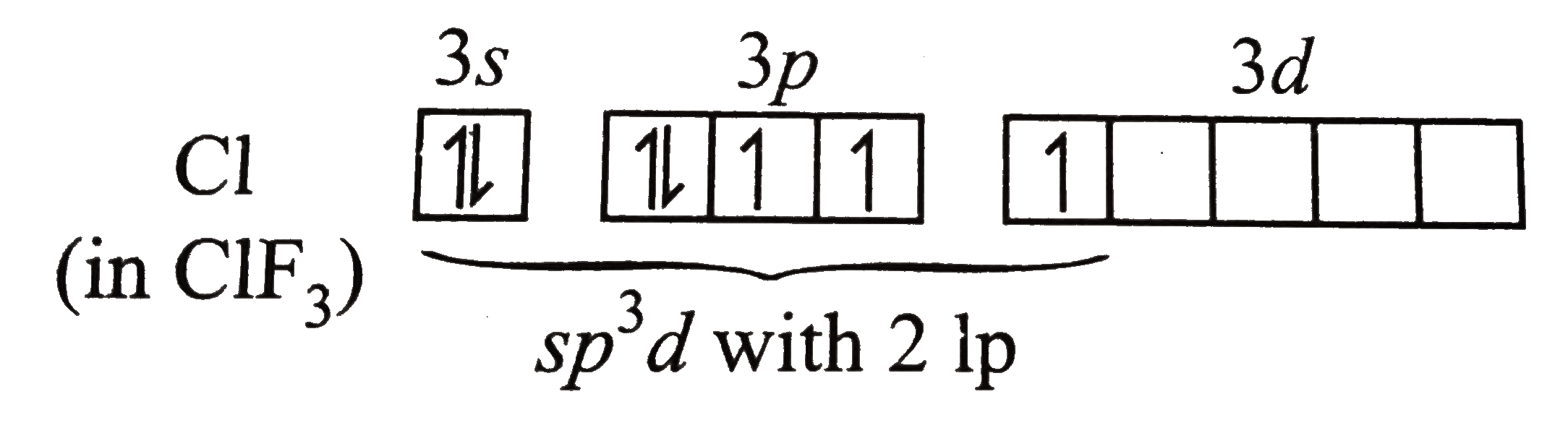
to 3 and shows an oxidation state of +3 and ClF3 exists. Whereas, F due to absence of d-orbital in its valence shell can have coordination number 1 only. Hence , FCl3 does not exist.
|
Similar Questions
Knowledge Check
Assertion (A): ClF3 exist but FCl3 does not exist.
Reason (R): ‘p does not have d-orbitals whereas 'Cl' has d-orbitals.ABoth A and R are true and R is the correct explanation of ABBoth A and R are true but R is not the correct explanation of ACA is true but R is falseDA is false but R is true
Give reason to explain why ClF3 exists but FCl3 does not exist.
View SolutionGive one reason to explain why ClF3 exists but FCl3 does not exist?
View SolutionKHF2 exists while KHCl2 does not. Explain.
View SolutionIF7 exists but BrF7 does not, why ?
View Solution(a). A strip of Cu dissolved in FeCl3, however Cu cannot displace Fe.
(b). [CuCl4]2− exists but [Cul_(4)]^(2-) does not. Why?
View SolutionGive reason to explain why ClF3 exists but FCl3 does not exist.
View SolutionOsO4 exists but OsF8 does not exist. Why?
View Solution

