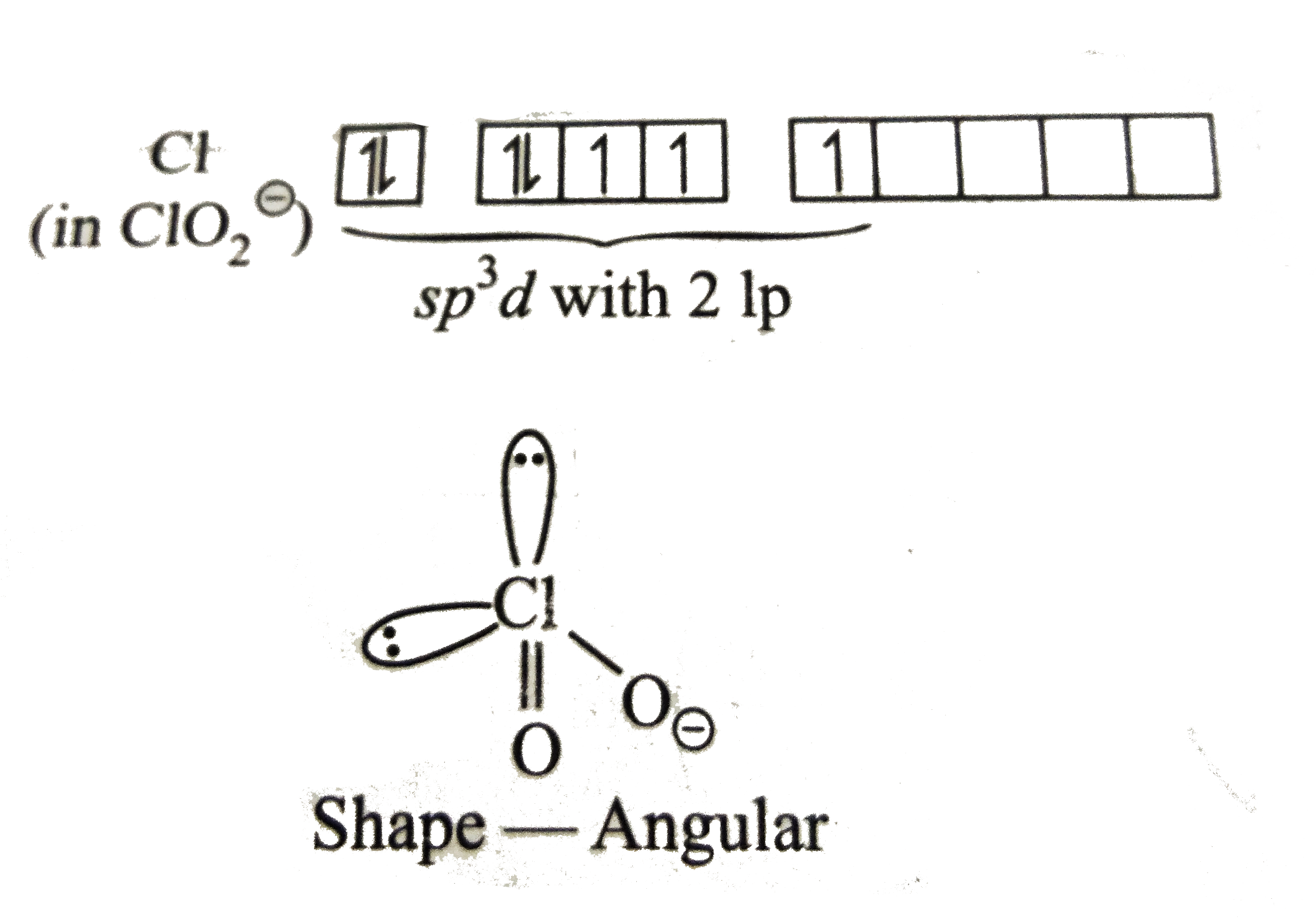Oxygen is more electronegative than chloride. In the series of oxyacids HOCI, HCIO2, HCIO3 and HCIO4, an increasing number of oxygen atom is bonded to the chlorine atom. Chlorine forms a number of oxyacids which differ in their stability order.
The hybridisation of CI in CIO2 and its shape are
A
sp3, pyramidal
B
sp2, angular
C
sp3, angular
D
sp2, trigonal planar
Video Solution
Text Solution
The correct Answer is:C
|
Similar Questions
Knowledge Check
Oxygen is more electronegative than chloride. In the series of oxyacids HOCI, HCIO2, HCIO3 and HCIO4, an increasing number of oxygen atom is bonded to the chlorine atom. Chlorine forms a number of oxyacids which differ in their stability order.
The order if acid strength og HOCI, HCIO2, HCIO3 and HCIO4 areAHCIO4>HCIO3>HCIO2>HOCIBHOCI>HCIO2>HCIO3>HCIO4CHCIO3>HCIO4>HCIO2>HOCIDHCIO3>HCIO2>HOCI>HCIO4Oxygen is more electronegative than chloride. In the series of oxyacids HOCI, HCIO2, HCIO3 and HCIO4, an increasing number of oxygen atom is bonded to the chlorine atom. Chlorine forms a number of oxyacids which differ in their stability order.
The least stable oxo-anion among the following isACIOΘBCIOΘ2CCIOΘ3DCIOΘ4Oxygen is more electronegative than chloride. In the series of oxyacids HOCI, HCIO2, HCIO3 and HCIO4, an increasing number of oxygen atom is bonded to the chlorine atom. Chlorine forms a number of oxyacids which differ in their stability order.
Which of the following is the strongest conjugate base ?ACIOΘBCIOΘ2CCIOΘ3DCIOΘ4
The atomic number of tin is 50 and chlorine is 17. What should be the shape of SnCl2 molecule in its vapour state?
View SolutionIn[ZnBr4]2− electron pairs in sp3 hybrid orbitals of the zinc atom form bonds to the bromineatoms Determine the number of unpaired electrons in the complex .
View SolutionWhich has the highest number of oxygen atoms?
1gO2or1gO3or1gO
View SolutionThe number of oxygen atoms required to combine with 7 g of N2 to form N2O3 when 80% of N2 is converted to N2O3.
View SolutionThe number of lone pairs on chlorine atom in CIO−,CIO−2,CIO−3,CI−4
View Solution