Write hydrate isomers of the following and also give increasing order of the conductance of the isomers
[Cr(H2O)6]Br3
(b) [Cu(H2O)4]CI2 .
Video Solution
Text Solution
Hydrate isomers of [Co(H2O)6]Br3 are
(i) (A)[Cr(H2O)6]Br3
(ii) (B)[Cr(H2O)5Br]Br2.H2O
(iii) (C)[Cr(H2O)4Br2]Br.2H2O
(iv) (D)[Cr(H2O)3Br3].3H2O
On ionisation the above hydrate isomers give
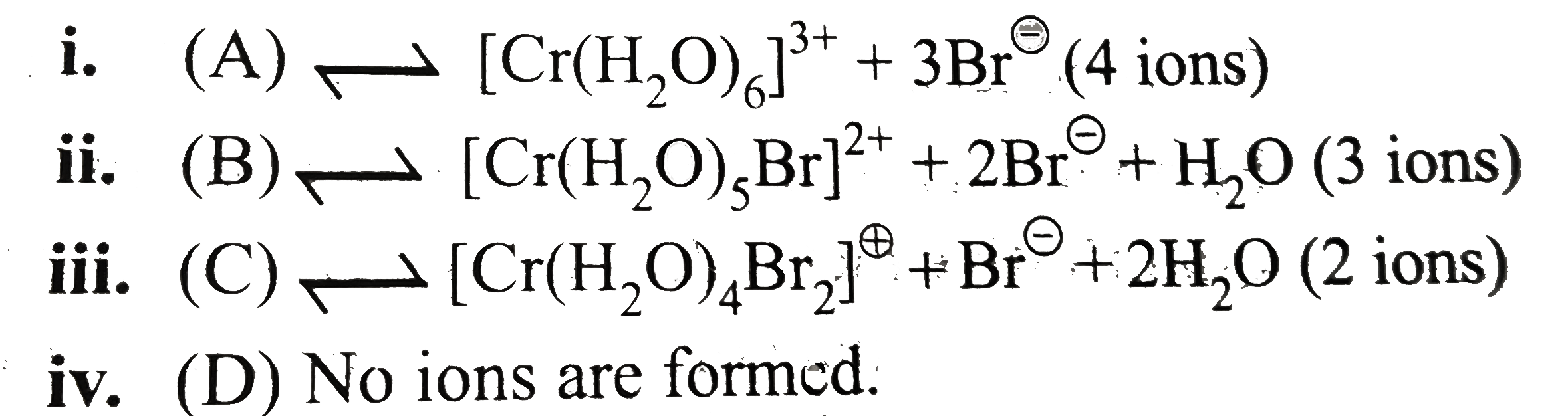
Greater the number of ions formed greater is the conductane Hence, increasing order of conductance is
D<C<B<A
(b) Hydrate isomers of [Cu(H2O)4]CI2 are
(i) (A)[Cu(H2O)3CI].H2O
(ii) (B)[Cu(H2O)2CI2].2H2O
(iii) (C)[Cu(H2O)4]CI2
On ionisation the above hydrate isomers give
(i) (A)→[Cu(H2O)3CI]⊕+CI⊕(2ions)
(ii) (B)→Noions
(iii) (C)→[Cu(H2O4]2++2CIΘ(3ions)
Greater the number of ions formed greater is the conductance. Hence order of conductance is B<A<C .
|
Similar Questions
Knowledge Check
The complex [Cr(H2O)5Cl]Brand[Cr(H2O)5Br]Cl show
ALinkage isomerismBIonisation isomerismCHydrate isomerismDCo-ordination isomerismThe number of hydrate isomers of CrCl36H2O are
A1B2C3D4Which of the following complex is not hydrate isomer of [Cr(H2O)6]Cl3 ?
A[Cr(H2O)3Cl3].3H2OB[Cr(H2O)5Cl]Cl2.3H2OC[Cr(H2O)4Cl2]Cl.2H2ODNone of these
Name an ionisation isomer of [Cr(H2O)5Br]SO4.
View SolutionNumber of optical isomers of [Cr(C2O4)3]3−
View SolutionIndicate the types of isomerism exhibited by the following complexes and draw the structures of these isomers
(i) K[Cr(H2O)2(C2O4)2]
(ii) [Co(NH3)5(NO2)](NO3)2
(iii) [Pt(NH3)(H2O)CI2]
View SolutionWhich of the following complex is not hydrate isomer of [Cr(H2O)6]Cl3 ?
View SolutionThe number of hydrate isomers of CrCl3⋅6H2O are
View Solution
