Megnetic moment value of Fe(H2O)5(NO)]2+ ion in 3.89B.M Find the out the oxidation state of iron and type of hybridisation .
Video Solution
Text Solution
νs=3.89BM corresponds to n=3
Thake 3.89≈4⇒√16≈√15B.M
The value √15BM corresponds to n=3
The oxidation state Fe in the complex
[Fe+1(H2O)5+1NO]2+ is +1 and CNofFe⊕=6
Fe(Z=26)⇒3d64s2,Fe⊕=3d64s1
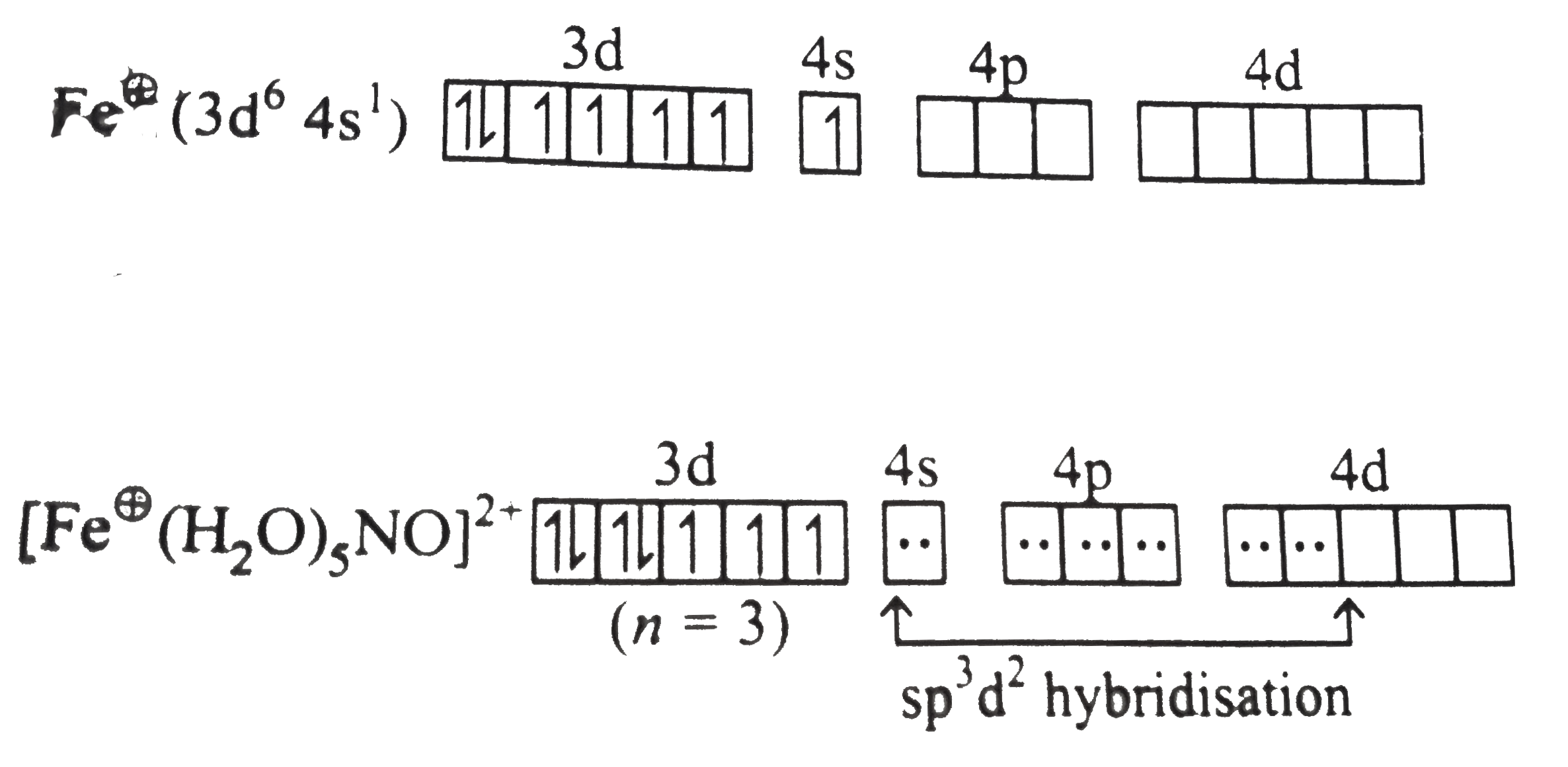
Since NO−+ is a strong ligand and H2O is a weak ligand so only one pairing of 3d and 4s electrons occurs which also corresponds n=3
(i) Oxidation state of Fe=+1
(ii) Hybridisation =sp3d2
(iii) Geometry = octahedral
(iv) Outer orbital complex .
|
Similar Questions
Knowledge Check
In the complex ion [Fe(H2O)5NO]2+
AFe is in the =2 oxidation state and NO coordinates as NO+BFe is in the +3 oxidation state and NO coordinates as NO−CFe is in the +2 oxidation state and NO coordinates as neutral NO (nitrosyl) radicalDFe is in the +1 oxidation state and NO coordinates as NO+ (nitrosonium ion)The oxidation state of Fe in brown complex [Fe(H2O)5NO]SO4 is
A+1B+2C+4D+3The oxidation state of iron in [Fe(H2O)5NO]2+ is
A1B2C3D4
Magnetic moment value of [Mn(CN)6]3− ion in 2.8BM Predict the type of hybridisation and geometry of the ion .
View SolutionThe brown ring complex compound is formulated as [Fe(H2O)5NO]SO4. The oxidation state of Fe is
View SolutionWhat is the oxidation number of Fe in [Fe(H2O)5(NO)]2+ ion?
View SolutionThe brown ring complex compound is formulated as [Fe(H0O)5(NO)]SO4 the oxidation state of iron is -
View SolutionThe magnetic moment (B.M) of Fe2+ ion is
View Solution
