Distinguish between the possibillities in complex ions of Δ=0 and CFSE=0 Give an example of each .
Video Solution
Text Solution
Δ=0 means no crystal field spliting i.e case of free gaseous ion CFSE=0 means equal occupancy of all the d-orbitals d5 weak field and d10 both weak and strong field ion Example of case when Δ0=0,Zn2+ gaseous ion CFSE=0∈[Mn(H2O)6]2+ and [Zn(H2O)6]2+
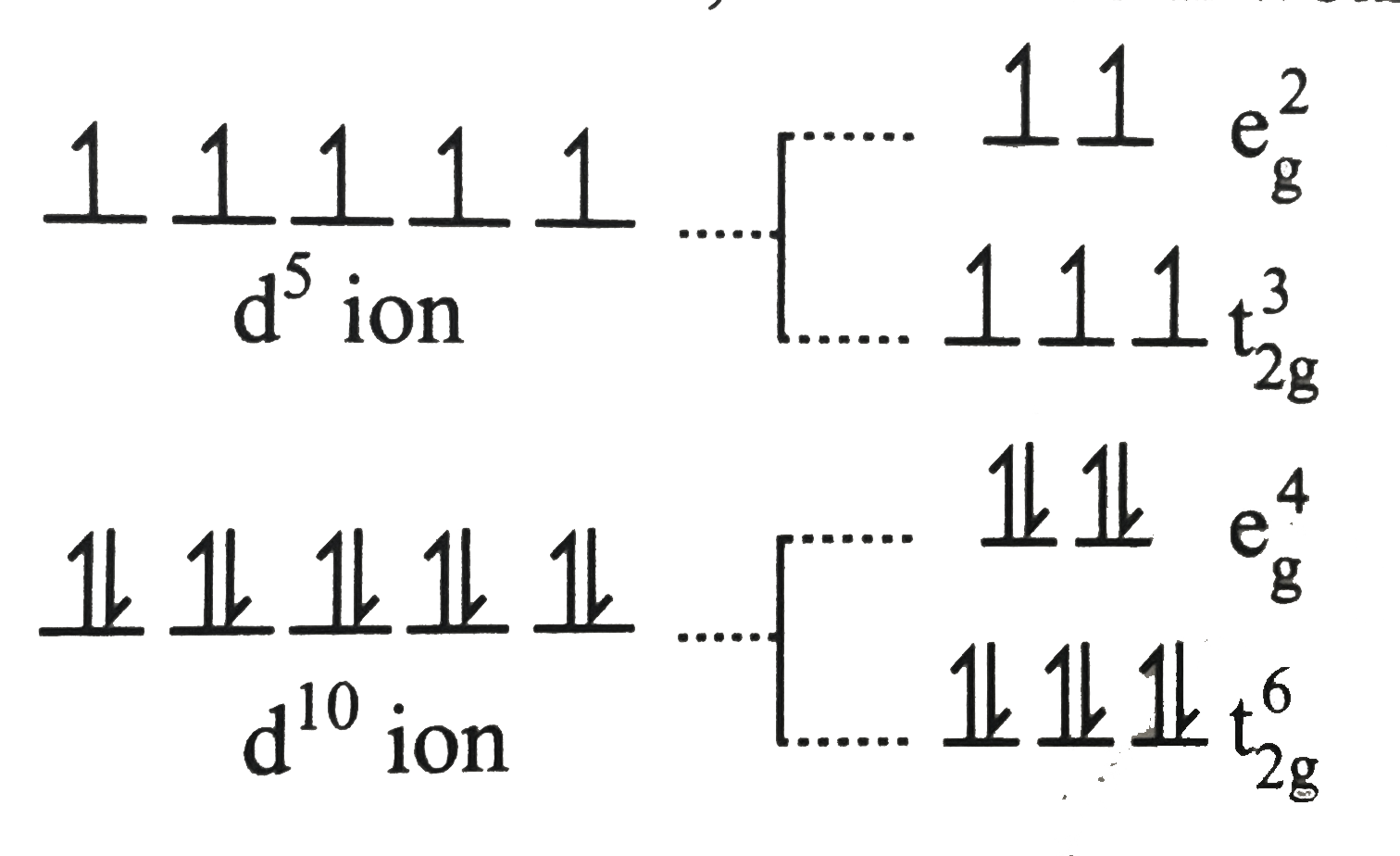 .
.
|
Similar Questions
Distinguish between isotopes and isobars, giving one example for each.
View SolutionThe complex ion having minimum magnitude of Δo(CFSE) is
View SolutionDefine complex ion, Give example.
View SolutionDistinguish between a prokaryote and a eukaryote, giving an example of each.
View SolutionDistinguish between mutlimolecular, macromolecular and associated colloids. Give one example of each.
View SolutionDistinguish between the possibillities in complex ions of Δ=0 and CFSE=0 Give an example of each .
View SolutionDistinguish between lobopodium and filopodium. Give an example to each of them.
View SolutionCFSE for the complex ion [Mn(CN)_(6)]^(3-) is
View Solution

