Write the IUPAC nomenclature of the given complex along with its hybridisation and structure
Ca[Cr(NO)(NH3)(CN)4],μsπn=1.73BM .
μspin of the complex is 1.73BM
μ=√n(n+2)=1.73⇒n=1
Thus chromium ion has one unpaired electron
Case I If NO is neutral
2+Co[2+Cr0NO(0NH3)−4(CN)4]2−
Then Cr2+ under the influence of strong field ligand (CNΘ) , it would correspond to 2 unpaired electrons
Cr(Z=24)⇒3d54s1,Cr2+⇒3d44s1
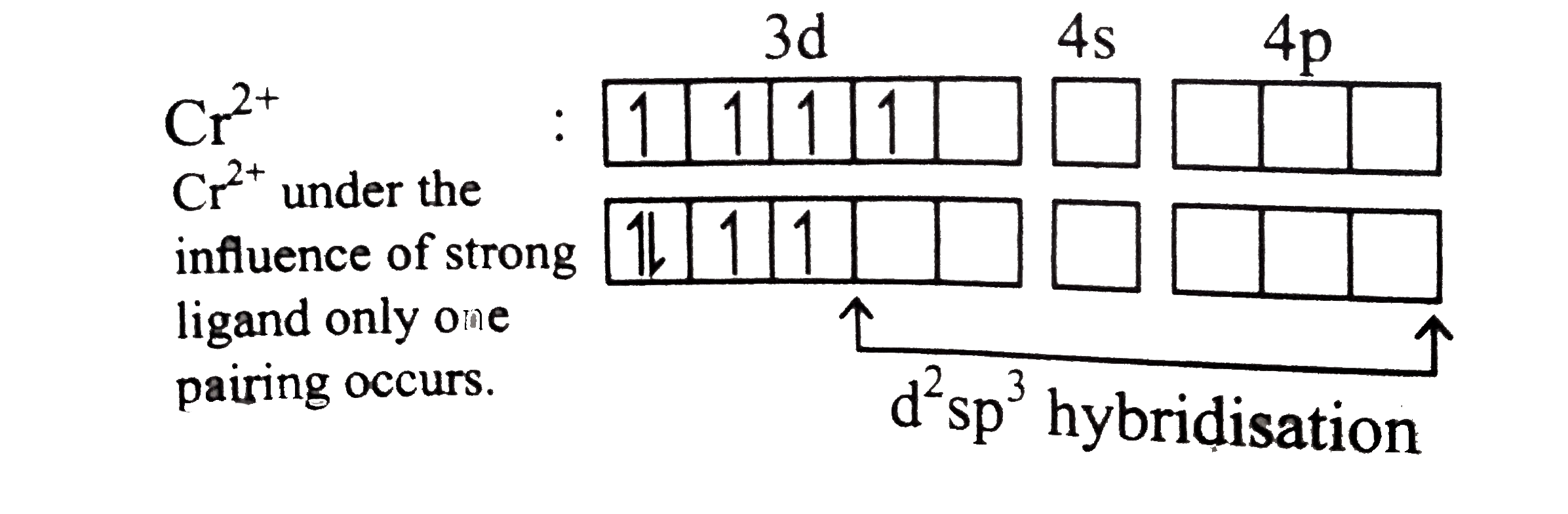
But the complex shows spin magnetic moment (μ) which corresponds to one unpaired electron
So,the ligand NO is +1 charged and teh IUPAC name of the compounds is Calcium amminetetracyano nitrosonium chromate(I)
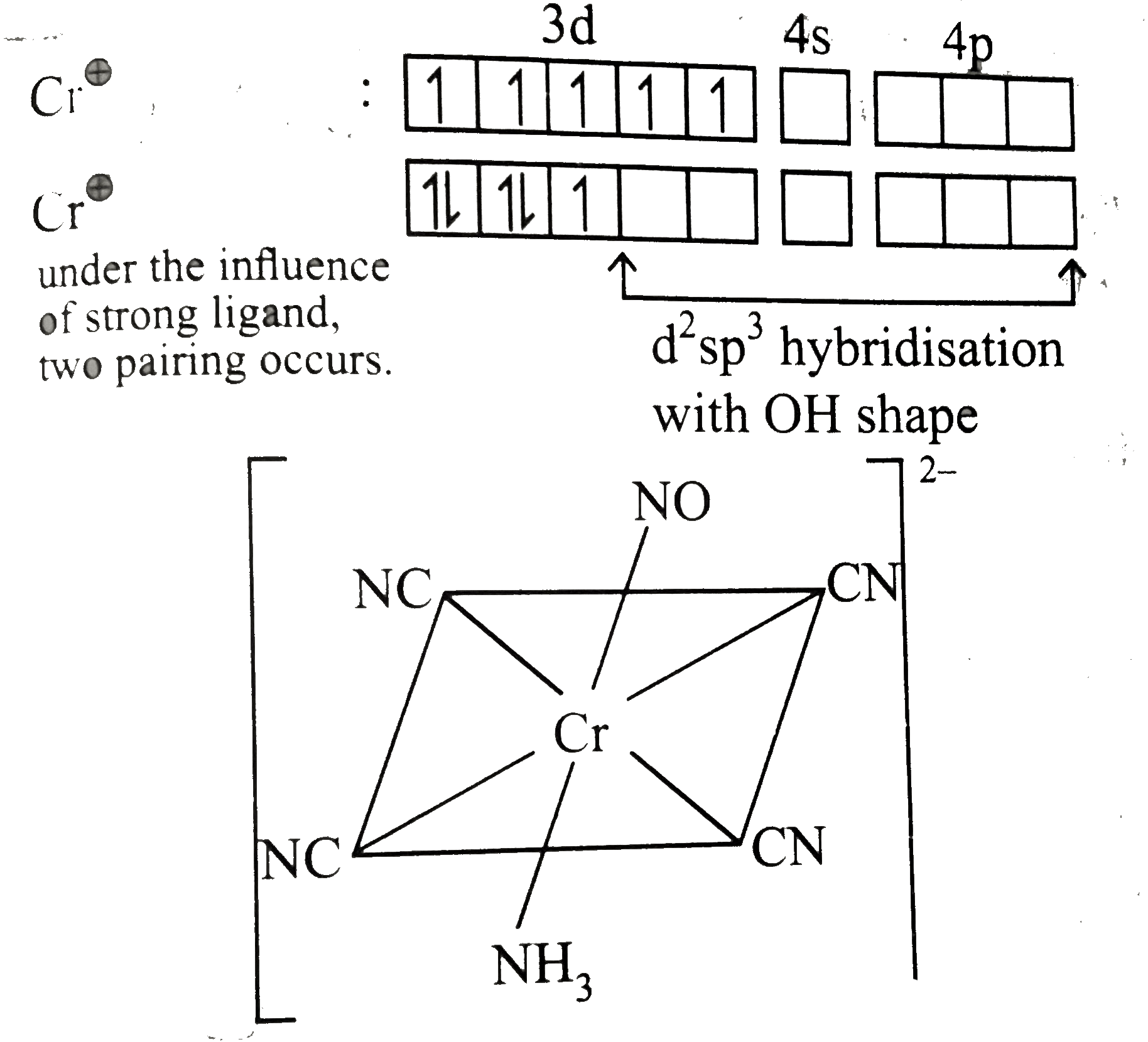 .
.
Similar Questions
Write the IUPAC nomenclature of the given complex along with its hybridization and structure K2[Cr(NO)(NH3)(CN)4],μ=1.73BM
View SolutionWrite the IUPAC nomenclature of the given complex along with its hybridisation and structure
K2[Cr(NO)(NH3)(CN)4],μ=1.73.BM .
View SolutionWrite the IUPAC nomenclature of the given complex along with its hybridisation and structure
K2[Cr(NO)(NH3)(CN)4],μ=1.73.BM .
View SolutionWrite the IUPAC nomenclature of the given complex along with its hybridisation and structure
K2[Cr(NO)(NH3)(CN)4],μ=1.73.BM .
View SolutionWrite the IUPAC name of the complex [Cr(NH3)4Cl2]Cl.
View SolutionWrite the IUPAC names of the complex K3[Cr(C2O4)3]
View SolutionWrite the IUPAC name of the complex [Cr(NH3)4Cl2]+.
View SolutionWrite the IUPAC name of the complex
(i) [Ag(NH3)2][Ag(CN)2]
View SolutionWrite IUPAC name of the complex:
Na3[Cr(OH)2F4]
View SolutionWrite the IUPAC name and hybridisation of the complex [Fe(CN)6]3−
View Solution
