Which of the following complexes diamagnetic:
A
[AuI4]Θ
B
[Co(H2O)6]3+
C
[CoI6]3−
D
[Co(CO)4]Θ
Video Solution
Text Solution
The correct Answer is:A, D
Au3+ (ground state)
Although CIΘ is a weak ligand but due to high Zeff value of Au3+ unpaired electrons will pair up
Hybridisation dsp2 shape square planar (n=0) μeff=0(Diamagnetic)
(b) [CoIII(H2O))6]3+,(Co3+=3d6)(weak field ligand)
(n=4) Hybridisation sp3d2μeff=√24BM
(Paramagnetic)
(c ) [CoIIII6]3−,(CO3+=3d6) (weak field ligand)
(n=4) Hybridisation sp3d2,μeff=√24BM
(Paramagnetic)
(d) [Co−1(CO)4]Θ,Co−1=3d84s2 (strong field ligand)
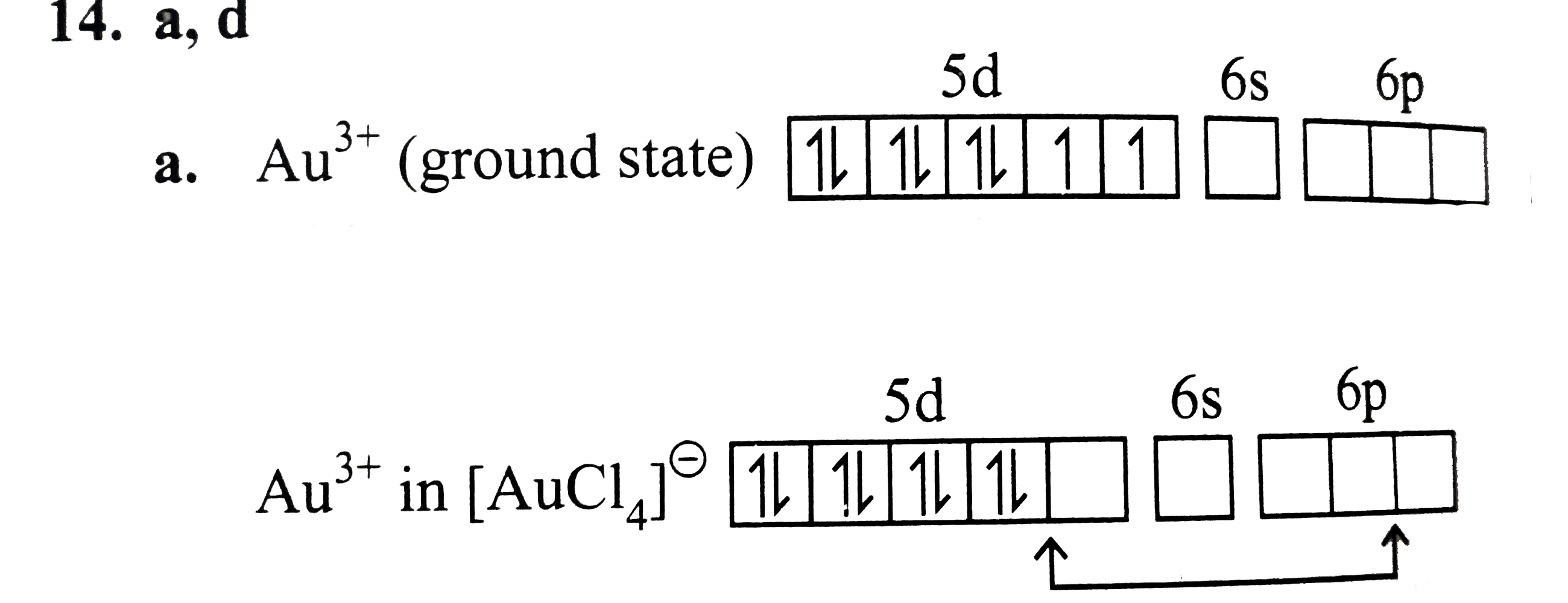
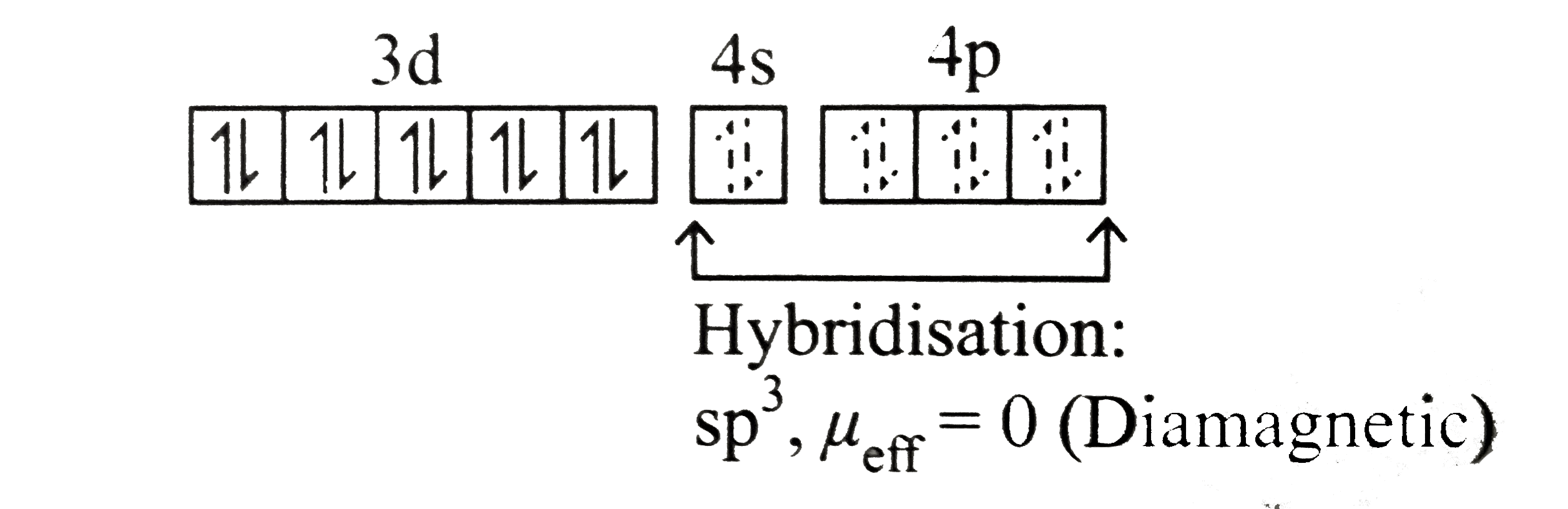
|
Similar Questions
Knowledge Check
Which of the following complex is diamagnetic ?
A[Co(CN)6]3−B[Mn(H2O)6]2+C[Fe(_(2)O)6]2+D[Fe(H2O)6]3+Which of the following complexes is diamagnetic ?
A[Fe(CN)6]4−B[Cu(NH3)4]2+C[Ti(H2O)6]3+D[Fe(CN)6]3−Which of the following complexes is diamagnetic ?
A[Fe(CN)6]4−B[Cu(NH3)4]2+C[Ti(H2O)6]3+D[Fe(CN)6]3−
Which of the following specie is diamagnetic in nature ?
View SolutionWhich of the following would be diamagnetic?
View SolutionWhich of the following would be diamagnetic?
View SolutionWhich of the following atom is diamagnetic?
View SolutionWhich among the following complexes is diamagnetic?
View Solution
