Which of the pair of complex compounds are tettrahedral as well as diamagnetic ? .
A
a.[CoCl4]Θ and [Co(CO)4]Θ
B
b.[Ag(SCN)4]2− and [NiCl4]2−
C
c.[Co(CO)4]Θ and [Ni(CN)4]4−
D
d.[PdCl4]2− and [Ni(CN)4]2−
Video Solution
Text Solution
The correct Answer is:C
Refer to Solution of Q.14 part d (Multiple Correct Answer Type).
|
Similar Questions
Knowledge Check
Which does not form complex compound ?
ANBPCAsDBiWhich does not form complex compounds with
AAgBCuCCdDPbWhich of the following complex compound is low spin, inner orbital , diamagnetic complex ?
A[Ni(NH3)6]Cl2BK3[Fe(CN)6]CK2[PtCl6]D[Cr(H2O)6]Cl3
Which of the following in/are inner orbital complex(es) as well as diamagnetic in nature?
[ Atomic Number : Cr=24, Mn=25, Fe=26, Co=27]
View SolutionWhich of the complex is inner orbital as well as diamagnetic in nature ?
View SolutionWhich one of the following is an inner orbital complex as well as diamagnetic in nature?
View SolutionWhich of the following is an inner orbital complex as well as diamagnetic in behaviour
[Atomic numbers Zn = 30, Cr = 24, Co = 27, Ni = 28.]
View SolutionWhich one of the following is an inner orbital complec as well as diamagnetic in behaviour?
View Solution

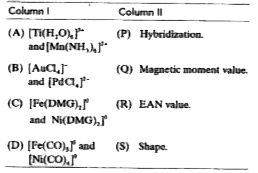 .
.