Give the total number of possible structural isomers of the compound II(CU)(NH3)4[IIPt14] .
Video Solution
Text Solution
The correct Answer is:4
The four isomers are
(i) [Cu(NH3)4][PtCI4]
(ii) [Cu(NH3)3CI][PtCI3(NH3)]
(iii) [Pt(NH3)3CI][CUCI3(NH3)]
(iv) [Pt(NH3)4][CuCI4]
|
Topper's Solved these Questions
- COORDINATION COMPOUNDSBook:CENGAGE CHEMISTRYChapter:COORDINATION COMPOUNDSExercise:Exercises Integer (Hybridisation , Vbt , Cft And Application)
Explore 13 Videos
- COORDINATION COMPOUNDSBook:CENGAGE CHEMISTRYChapter:COORDINATION COMPOUNDSExercise:Exercises Fill The Blanks
Explore 32 Videos
- COORDINATION COMPOUNDSBook:CENGAGE CHEMISTRYChapter:COORDINATION COMPOUNDSExercise:Exercises Integer (Naming And Terminology)
Explore 16 Videos
- CHEMICAL KINETICSBook:CENGAGE CHEMISTRYChapter:CHEMICAL KINETICSExercise:Archives Subjective
Explore 23 Videos
- D AND F BLOCK ELEMENTSBook:CENGAGE CHEMISTRYChapter:D AND F BLOCK ELEMENTSExercise:Archives Subjective
Explore 29 Videos
Similar Questions
Knowledge Check
The total number of possible isomers of the compound [CuII(NH3)4][PtIICl4] are :
A3B5C4D6
The total number of optical isomers possible for this compound are?A9B8C10D16The total number possible isomers for the complex compound [CuII(NH3)4[PtIICI4] are
A3B6C5D4
Give the total number of possible isomers of
[Co12(CN)(NH3)en] .
View SolutionThe total No. of possible isomers of the compound [Cu∣∣(NH3)4][Pt∣∣Cl4] are:-
View SolutionThe total number of geometrical isomers possible in the following compound is
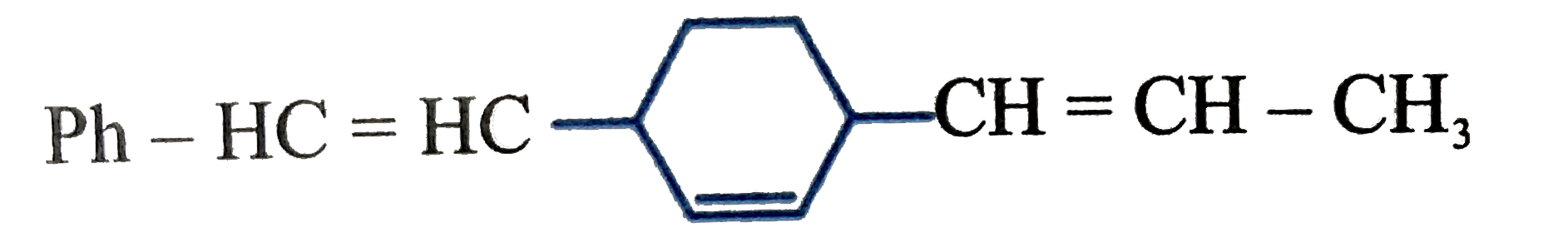
View SolutionThe total number possible isomers for the complex compound [CuII(NH3)4[PtIICI4] are
View SolutionThe total No. of possible isomers of the compound [CuII(NH3)4][PtIICl4] are :-
View Solution
