[NiCI2{P(C2H5)2(C6H5)}2] exhibits temperature dependent magnetic behaviour(paramagnetic//diamagnetic) The coordination geometries of Ni2+ in the paramagnetic and diamagnetic states are respectively .
A
Tetrahedral and tetrahedral
B
Square planar and square planar
C
Tetrahedral and square planar
D
Square planar and tetrahedral
Video Solution
Text Solution
The correct Answer is:c
[NiCI2{Pet2Ph}2] contains Ni2+ ions =3d84s2
In high spin state in presence of weak ligand or at low temperature it is paramagnetic sp3 hybridised, tetrahedral In low spin state i.e in presence of strong ligands or high temperature it is diamagnetic (pairing occurs) dsp2 hybridised, square planar .
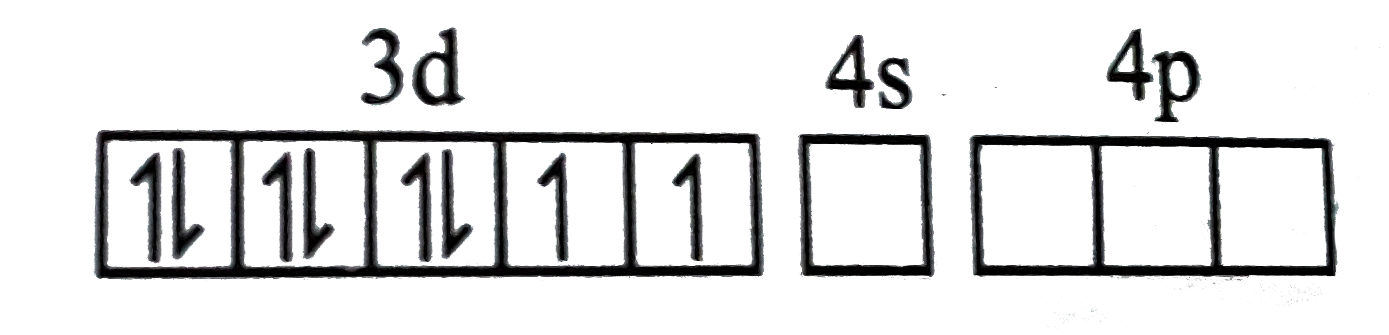
|
Similar Questions
Knowledge Check
[NiCl2{P(C2H5)2(C6H5)}2] exhibits temperature dependent magnetic behaviour. The coordination geometries of Ni2+ in the paramagnetic and diamagnetic states are:
Atetrahedral and tetrahedralBsquare planar and square planarCtetrahedral and square planarDsquare planar and tetrahedral[NiCl2{P(C2H5)2(C6H5)]2] exibits temperature dependent magnetic behaviour (paramagnetic/diamangetic). The coordination geometries of Ni2+ in the paramagnetic and diamagnetic states are respectively
Atetrahedral and tetrahedralBsquare planar and square planarCtetrahedral and square planarDsquare planar and tetrahedral[NiCl2{P(C2H5)2(C6H5)}2] exhibits temperature dependent magnetic behaviour. The coordination geometries of Ni2+ in the paramagnetic and diamagnetic states are:
Atetrahedral and tetrahedralBsquare planar and square planarCtetrahedral and square planarDsquare planar and tetrahedral
[NiCI4]2− is diamagnetic in nature .
View SolutionCh3−O−C3H7andC2H5−O−C2H5 exhibit which type of isomerism
View SolutionNl(CO)4is diamagnetic, [NlCl4]2− and [Ni(CN)4]2− are paramagnetic
View SolutionThe compounds CH3−O−C3H7 and C2H5−O−C2H5 exhibit
View SolutionThe geometries of [Ni(CO)4] and [NiCI2(PPh3)−(2)] are
View Solution

