Some pule green crystals are strongly heated .The gases then off are passed into a container surrounded by ice and then through a solution of acidified KMnO4 The KMnO4 is decolrised, a waxy white solid iws formed in the ice container this is dissolvesd in water .The solution will
Give a precipitate with silver nitric solution
Give a precipitate with burium chloride solution
Turn red litmus blue
Give blue colour with strach solution
The correct Answer is:b
Assume green crystal of FeSO4
FeSO4Δ−−→Fe2O3+SO2+SO3
SO3+MnOΘ4→Mn2−+SO2−4
SO3 forms waxy white solid (H2SO4) in ice container white is dissolved in water and gives SO2−4 ion .When BaCI2 reacts SO2+4 ion it forms ppt of BeSO4
Topper's Solved these Questions
- QUALITATIVE INORGANIC SALT ANALYSISBook:CENGAGE CHEMISTRYChapter:QUALITATIVE INORGANIC SALT ANALYSISExercise:Exercises (Single Correct) Part-B(Dry Test)
Explore 10 Videos
- QUALITATIVE INORGANIC SALT ANALYSISBook:CENGAGE CHEMISTRYChapter:QUALITATIVE INORGANIC SALT ANALYSISExercise:Exercises (Single Correct) Part-C (Analysis Of Cations)
Explore 43 Videos
- QUALITATIVE INORGANIC SALT ANALYSISBook:CENGAGE CHEMISTRYChapter:QUALITATIVE INORGANIC SALT ANALYSISExercise:Exercises (Multiple Correct) Part-D (Miscellaneous)
Explore 20 Videos
- P-BLOCK GROUP 18 ELEMENTS - THE INERT GASESBook:CENGAGE CHEMISTRYChapter:P-BLOCK GROUP 18 ELEMENTS - THE INERT GASESExercise:Ex 5.1 (Objective)
Explore 14 Videos
- REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSBook:CENGAGE CHEMISTRYChapter:REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSExercise:SUBJECTIVE TYPE
Explore 3 Videos
Similar Questions
The compound formed in the borax berax test of Cu2+ in oxidising flame is
View SolutionStrongly acidified solution of barium give a white precipitate with ……which did not dissolve even after large addition of water
View SolutionSelect the correct statement(s):
View SolutionBoiling point and melting point of noble gases are in the order
View SolutionWrite the statements of the products (A),(B),(C),(D), and (E) in the following scheme.
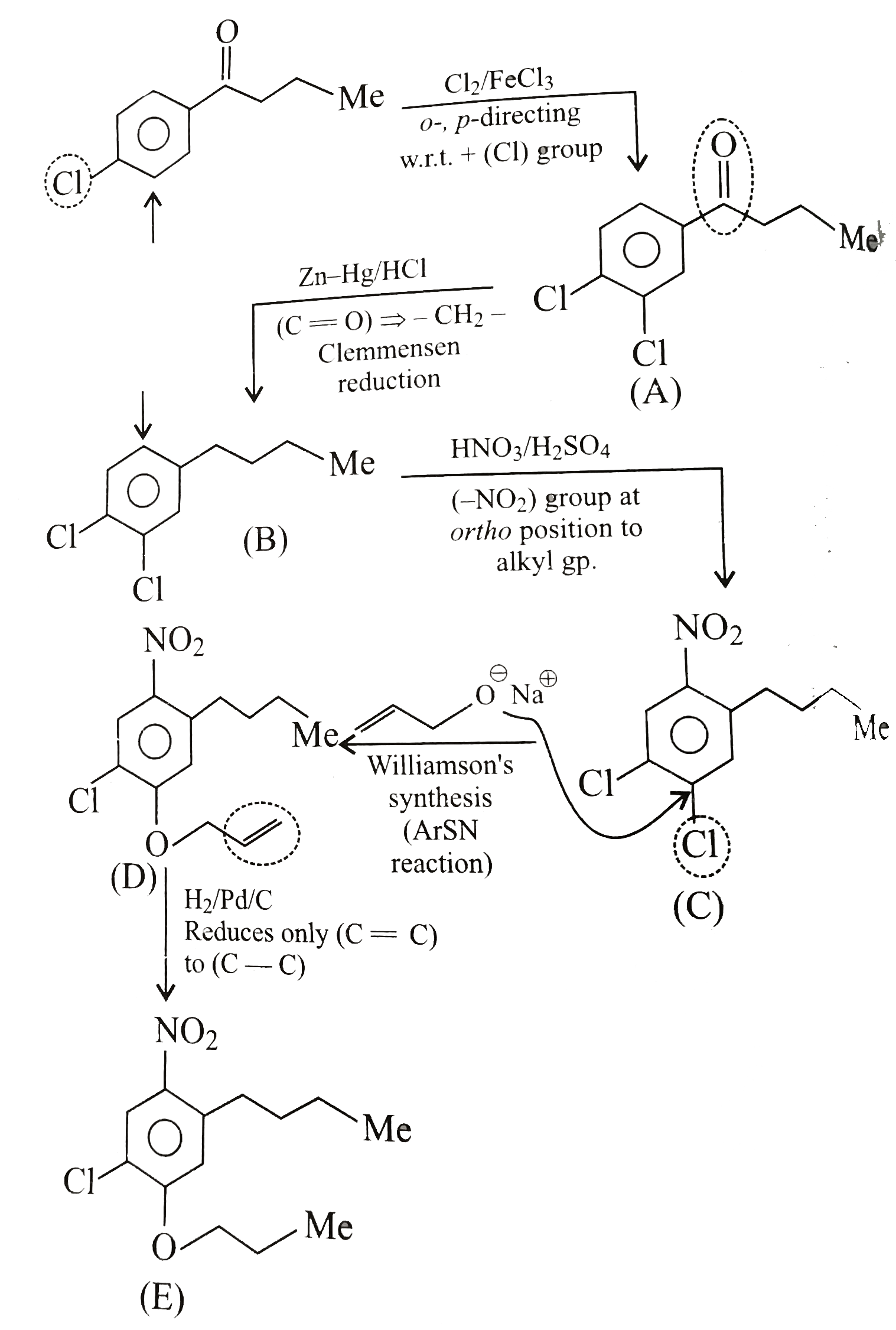
View Solution5 L of KMnO_(4) solution contains 0.01 equiv. of KMnO_(4) . 50 ml of the given solution contain, how many moles of KMnO_(4) ? KMnO_(4) rarr MnO_(2)
View SolutionBy passing H2S in acidified KMnO4 solution we get
View SolutionWhen H_(2)S is passed through nitric acid and acidified KMnO_(4) solution the product formed is
View SolutionA compound (A) forms a unstable pale blue colour solution in water. The solution decolourised Br_(2) water and an acidified solution of KMnO_(4) . The possible compound (A) is :
View SolutionSome pale-green crystals are strongly heated. The gases give off are passed into a container surrounded by ice and then through a solution of acidified KMnO4. The KMnO4 is decolourised , solution of green coloured crystal will
View SolutionWhen heated strongly , KMnO_(4) decomposes to form "…..................." and "….................."
View SolutionSolid KmnO4 on heating with H2 forms
View SolutionAcidified KMnO_(4) solution is decolourized by
View SolutionNO gas is passed through acidified KMnO4 solution.
View SolutionWhat happens when SO2 is passed in acidified KMnO4 solution ?
View Solution

