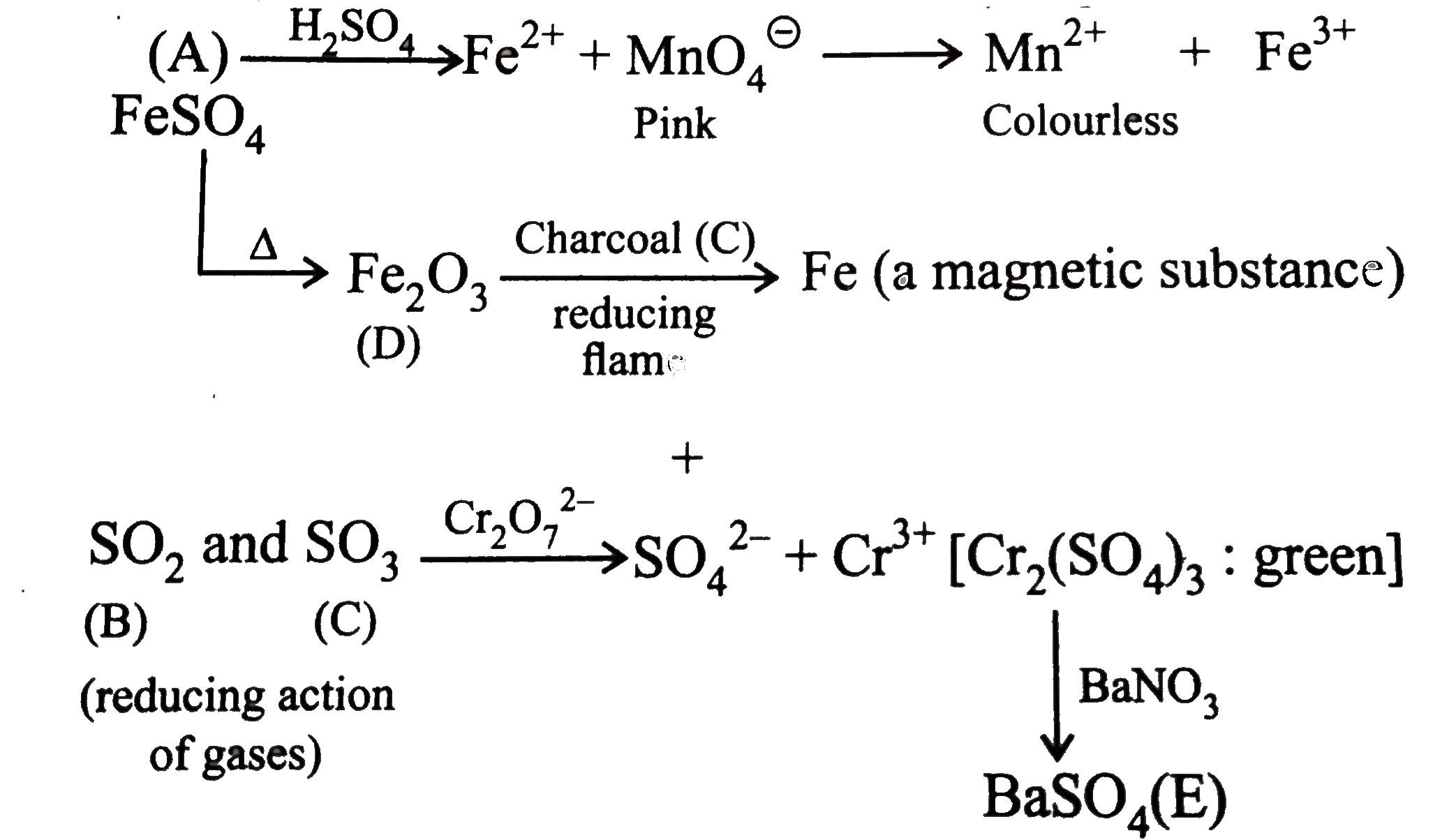
Compound A is the light crystaline solid .IT gives the following tests:
i. IT dissolves in dilute sulphuric acid, NO gas is produced
ii. A drop of MnO4 is added to the above solution .The pink colour disappears
iii. Compound A is heated strongly .Gases B and C , with pungent smell , come out A brown D is left behind
iv . THe gas mixture (B and C) is passed into a dchromate solution .The solution turn green
v. THe green solution from step (iv) gives a white precipitate E with a soluttion of barium nitrate .
vi. Residue D from step (iii) is heated on charcoal in a reducing flame it gives a magnetic subsytance .Name the compounds A,B,C, D and E
A: FeSo4:B:SO2,C:SO4,D:Fe2O3,E:7H2O
It is again a problem involving FeSO4,7H)2O
Topper's Solved these Questions
- QUALITATIVE INORGANIC SALT ANALYSISBook:CENGAGE CHEMISTRYChapter:QUALITATIVE INORGANIC SALT ANALYSISExercise:Ex 8.1
Explore 14 Videos
- QUALITATIVE INORGANIC SALT ANALYSISBook:CENGAGE CHEMISTRYChapter:QUALITATIVE INORGANIC SALT ANALYSISExercise:Ex 8.2
Explore 79 Videos
- QUALITATIVE INORGANIC SALT ANALYSISBook:CENGAGE CHEMISTRYChapter:QUALITATIVE INORGANIC SALT ANALYSISExercise:Exercises Archives (True/False)
Explore 2 Videos
- P-BLOCK GROUP 18 ELEMENTS - THE INERT GASESBook:CENGAGE CHEMISTRYChapter:P-BLOCK GROUP 18 ELEMENTS - THE INERT GASESExercise:Ex 5.1 (Objective)
Explore 14 Videos
- REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSBook:CENGAGE CHEMISTRYChapter:REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSExercise:SUBJECTIVE TYPE
Explore 3 Videos
Similar Questions
compound a is a light green crystalline solid. It gives the following tests
(i) It dissolves in dilute sulphuric acid. No gas is produced.
(ii) A drop of KMnO4 is added to the above solution. The pink colour disappears.
(iii) Compound A is heated strongly. Gases B and C, with pungent smell, come out. A brown residue D is left behind.
(iv) The gas mixture (B and C) is passed into a dichromate solution. The solution turns green.
(v) The green solution forms step (iv) gives a white precipitate E with a solution of barium nitrate.
(vi) Residue D from step (iii) is heated on charcoal in a reducing flame. It gives a magnetic substance.
Name the compound A, B, C, D and E.
View SolutionCompound (A) is a light green crystalline solid it gives the following tests
i. If dissolves in dilute sulphuric acid .No gas is produced
ii A drop of MnO4 is added to the above solution .The pink colour disappears
iii Compound (A) is heated atrongly green (B) and (C ) with pungent smell came out .A brown residue (D) is lrft behind
iv The gas mixture [(B)and(C)] is passed into a dichromate solution .THe solution turms green
THe green solution from step (iv) gives a white precipitate (E ) with a solution of barium nitrate
vi . Residue (D) from (v) is heated on charcoal in reducing flame it gives a magnetic substance Identify the compound (A) to (E )
View Solution
Knowledge Check
When an orange coloured crystallc compound [A] was heated with common salt and concentrated sulphuric acid , an orange red coloured gas [B] was evolved. The gas [B] on passing through NaOH solution gave a yellow solution [C] . The solution on reacting with an aqueous solution of lead acetate gave a yellow precipitate.
The crystalline compound [A] is:ACobalt nitrateBmanganese sulphateCPotassium dichromate.DAmmonium dichromate.When an orange coloured crystallc compound [A] was heated with common salt and concentrated sulphuric acid , an orange red coloured gas [B] was evolved. The gas [B] on passing through NaOH solution gave a yellow solution [C] . The solution on reacting with an aqueous solution of lead acetate gave a yellow precipitate.
This gas [B] is :AChlorineBbromineCchromyl chorideDnitrogen percipitate.BaCl2 solution gives a white ppt. with a solution of a slat, which dissolves in dil. HCl with the evolution of a colourless, pungent smelling gas. The radical in the salt is
ASO2−4BHSO−4CSO2−3DCO2−3
Write balanced equation for the following:
(i). NaCl is heated with sulphuric acid in the presence of MnO2.
(ii). Chlorine gas is passed into a solution of NaI in water.
View SolutionCompound (A) is greenish solid which gives the following tests : (i) Addition of BaCl2 solution to a solution of (A) results in the formation of a white precipitate (B) which is insoluble in dilute HCl . (ii) On heating water vapours and two oxides of sulphur (C ) & (D) are liberated,leaving a red brown residue (E ). (iii) Compound (E) dissolves in warm concentrated HCl to give a yellow solution (F). (iv) With H2S gas, the solution (F) yields a yellow white precipitate . (G) which when filtered leaves a greenish filtrate (H). Identify the compounds A to H.
View SolutionAn aqueous solution .A. turns phenolphthalein solution pink. On addition of an aqueous solution .B. to .A., the pink colour disappears. The following statement is true for solution .A. and .B..
View SolutionAn aqueous solution 'A turns phenol phthalein solution pink. On addition of an aqueous solution 'B' to 'A', the pink colour disappears. The following statement is true for solution 'A' and 'B'.
View SolutionAn inorganic compound A is dissolved in dilute hydrochloric acid and is then warmed. A colourless gas B is produced. When a filter paper moistened with potassium iodate and the strach solution is exposed to the gas it turns blue. The gas B and the compound A are:
View Solution

