Why does NH3 form hydrogen bond but PH3 does not?
Video Solution
Text Solution
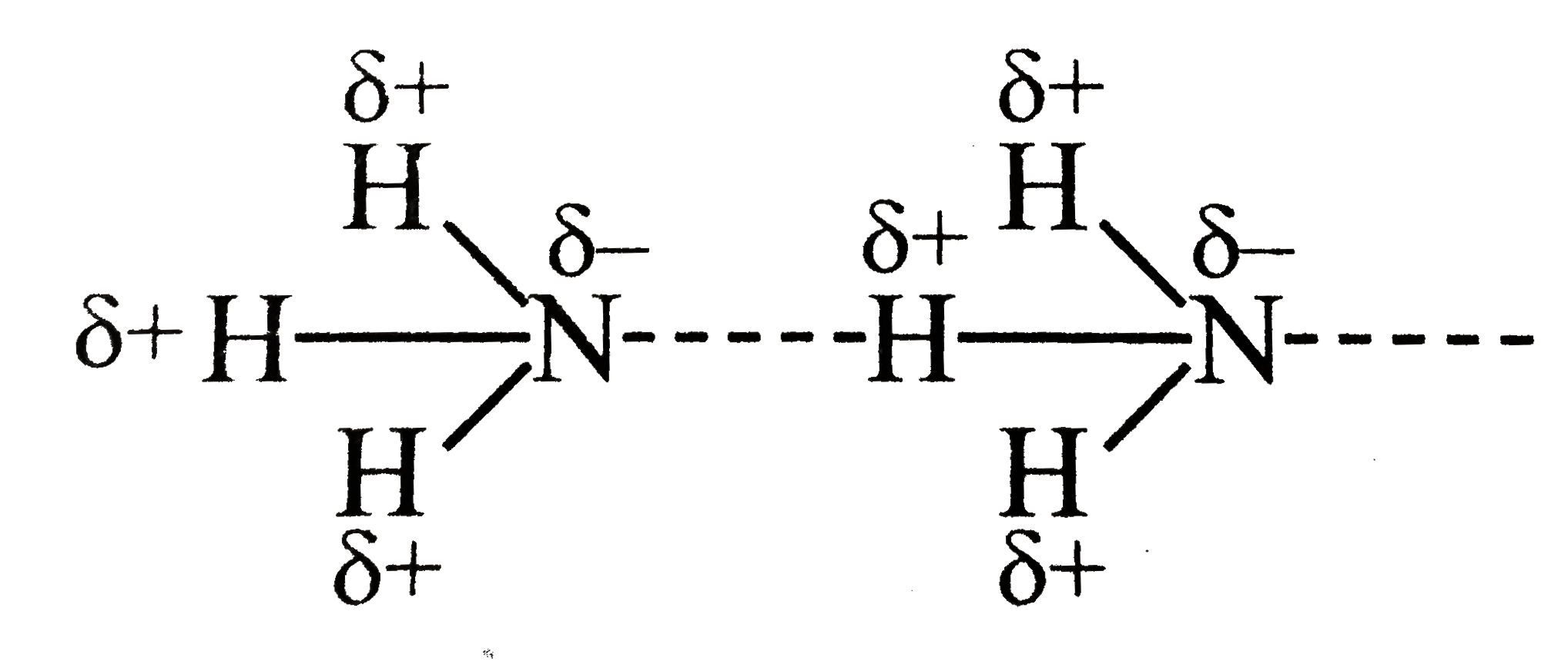
Nitrogen is more electronegative than phosphorous. Hence N−H is highly polar bond and NH3 undergoes intermolecular hydrogen bonding. On the other hand, polarity of P−H bind is very less and hence PH3 molecule does not form hydrogen bonds.
|
Similar Questions
Knowledge Check
Acetylene does not form hydrogen bond because :
AC is less electronegative than hydrogenBElectronegativity of C and H are exactly equalCCarbon does not have any lone pair of electronsDNone of the above
Why does NH3 form hydrogen bond but PH3 does not?
View SolutionWhy does NH4 form hydrogen bond but PH3 does not ?
View SolutionWhy does NH3 form hydrogen bonding while PH3 does not ?
View SolutionWhy does NH3 form hydrogen reactivity of group 15 elements.
View SolutionExplain why inspite of nearly the same electronegativity, oxygen forms hydrogen bonding while chlorine does not.
View SolutionExplain why inspite of nearly the same electronegativity, oxygen forms hydrogen bonding while chlorine does not.
View SolutionExplain why in spite of nearly the same electronegativity, oxygen forms hydrogen bonding while chlorine does not.
View Solution

