Why sulphur exhibits even oxidation states only?
Video Solution
Text Solution
Ground state electronic configuration of S is 3s2,3p4
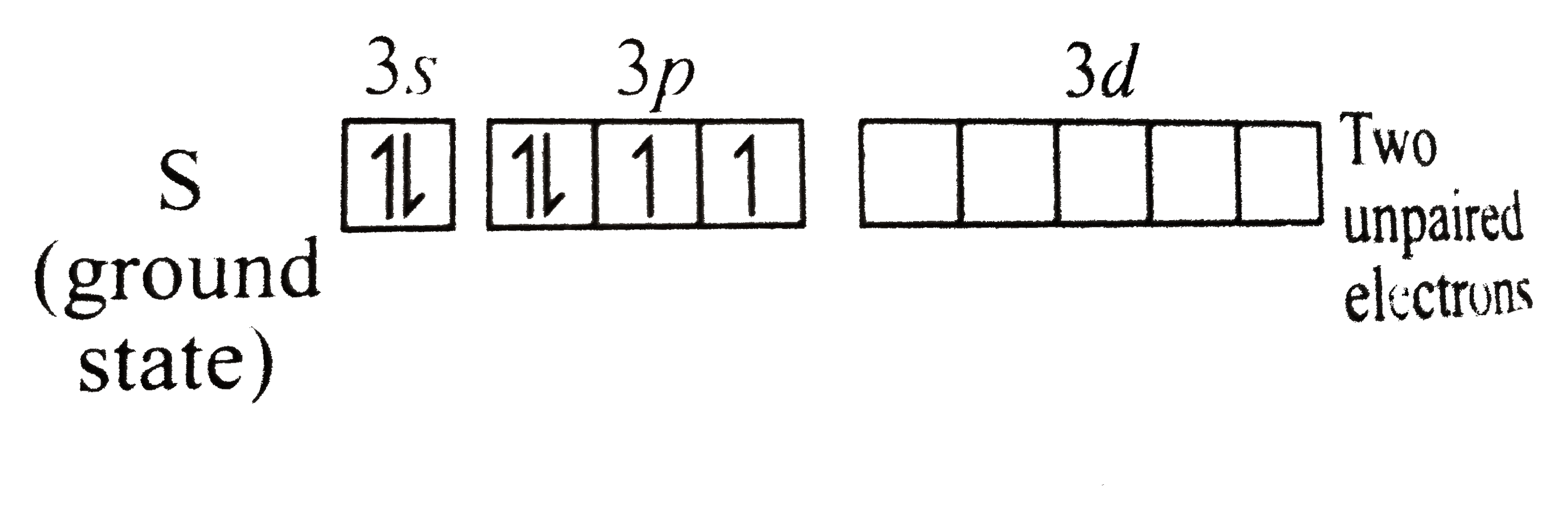
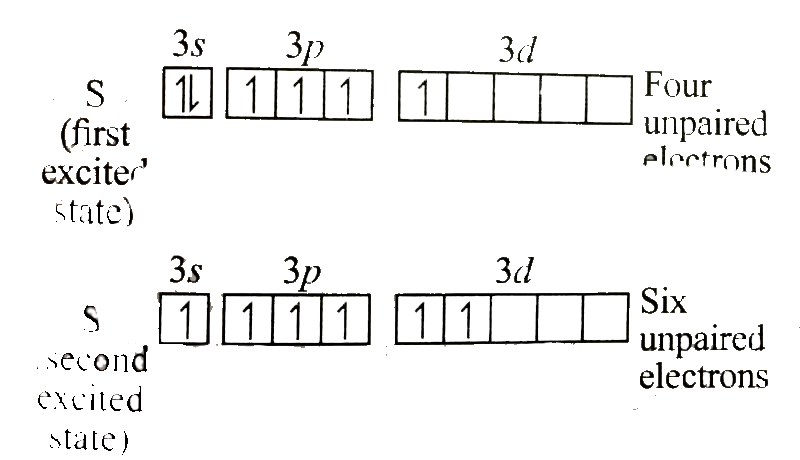
Since there are two four or six unpaired electrons that is why sulphur shows even oxidation states only.
|
Similar Questions
Knowledge Check
Actinides exhibits oxidation state
A+3and+4B+4and5C+5and+6D+2 to +7Sulphur has highest oxidation state in
ASO2BH2SO4CNa2S4O6DNa2S2O3Fluorine exhibits an oxidation state of only -1 because :
Ait can readily accept an electronBit is very strongly electronegativeCit is a non-metalDit belongs to halogen family

