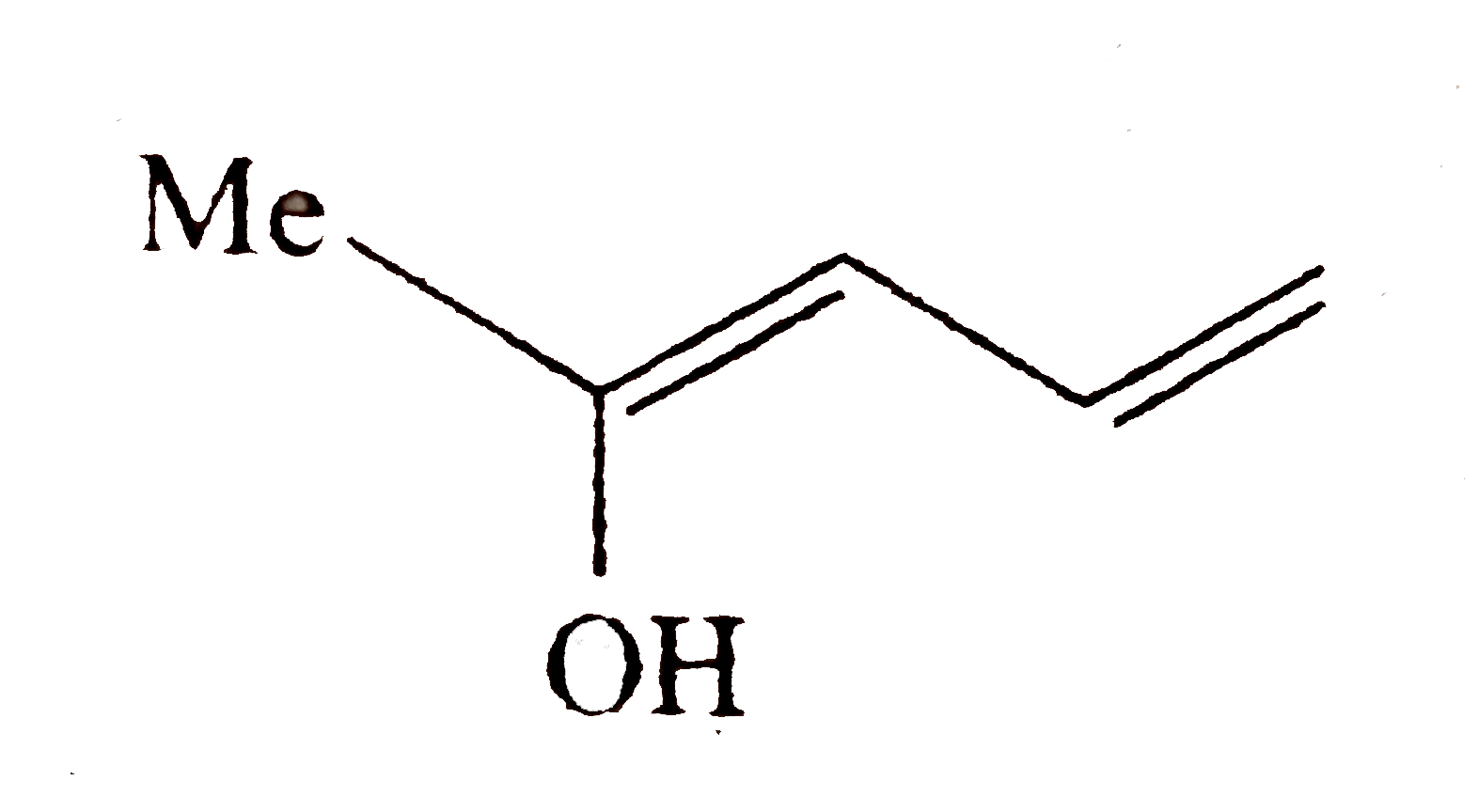
Write the correct resonance structure of the given compound.
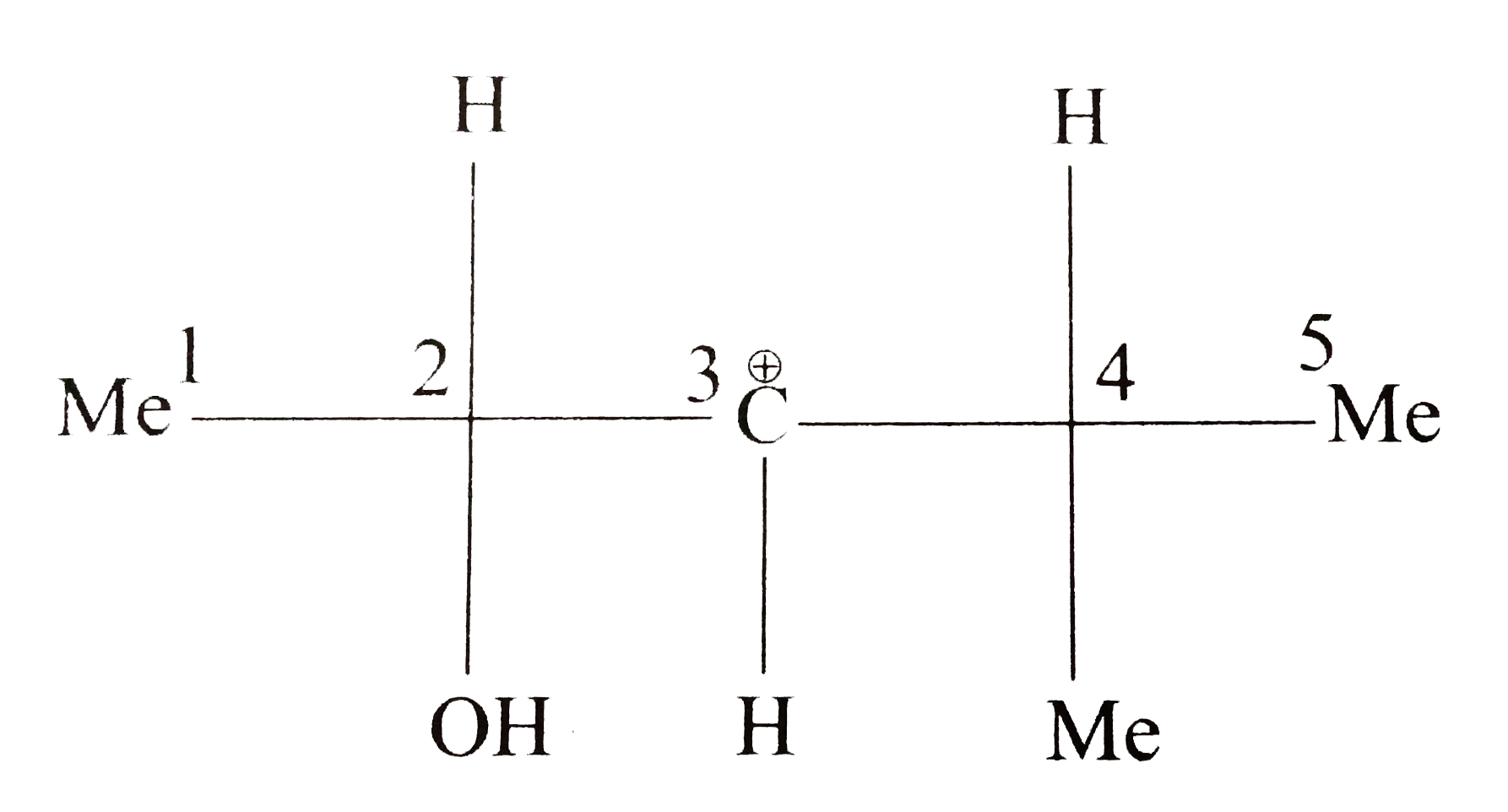 .
.
Text Solution
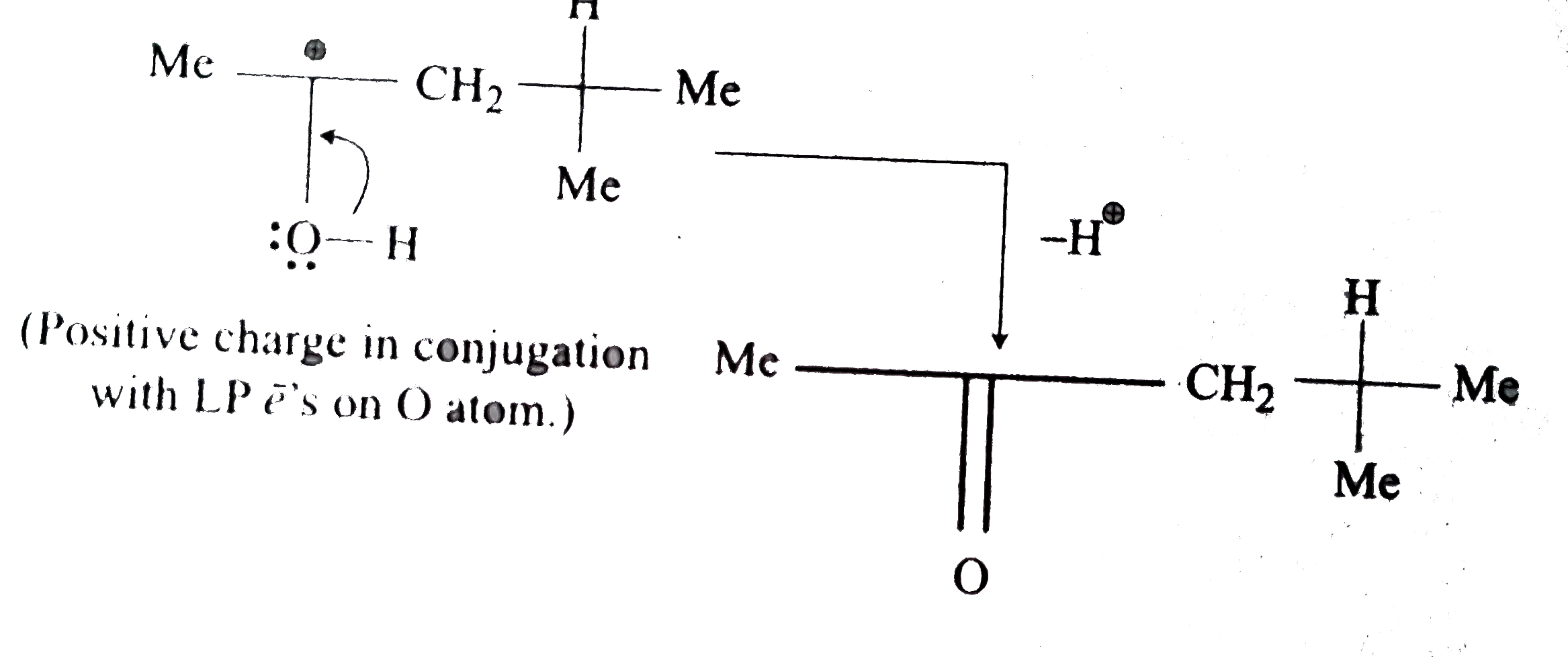
There are four possibilities, either HӨ shifts from C−2 or C−4 or Me shifts from C−2 or C−4. But HӨ shift produces more stable resonance structure.
Moreover, HӨ shift from C−2 would give more stable structure due ti conjugation of positive charge with the LP¯e,s on O atom of OH group.
 ,
,
 .
.
|
Similar Questions
Knowledge Check
Identify the correct resonance structures of carbon dioxide from the one given below:
AO−C≡OBO=C=OC.−O≡C−O+D.−O−C≡O+
Write resonating structure of the compound
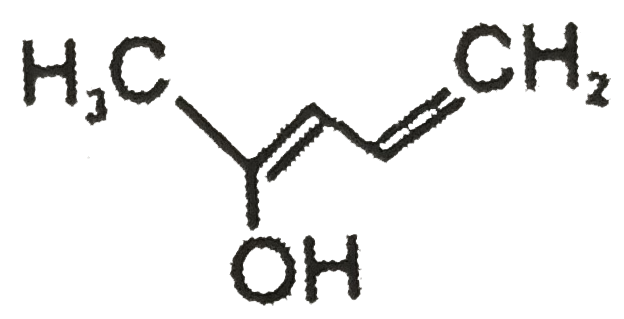
View SolutionWrite resonating structure of the compound

View SolutionWrite the resonance structure of CO2 .
View SolutionWrite the resonance structure of nitrous oxide.
View SolutionWrite the resonance structure of nitrous oxide.
View SolutionIdentify the correct resonance structures of carbon dioxide from the one given below:
View SolutionIdentify the correct resonance structures of carbon dioxide from the one given below:
View Solution