Arrange the following alcohols in the decreasing order of reactivity towards
(a) Lucas reagent
Sodium metal
(1) 1-Butano1
(2) sodium metal
(3) 2-Methy1-2-propanol.
Video Solution
Text Solution
(a) 3>2>1[with Lucas reagent (anhydrous ZnC12+conc.HC1(1:1),3∘>2∘>1∘ alcohol].
(b) 1>2>3(with sodium metal reactivity is 1∘>2∘>3∘ alcohol).
|
Similar Questions
Knowledge Check
Arrange the following metals in the decreasing order of reactivity:
ASodium > silver > iron > goldBGold > silver > iron > sodiumCIron > sodium > silver > goldDSodium > iron > silver > goldArrange the following metals in the decreasing order of reactivity:
AIron > sodium > silver > goldBSodium > silver > iron > goldCGold > silver > iron > sodiumDSodium > iron > silver > goldArrange the following compounds in the decreasing order of their reactivity towards SN2 reaction :
 Aiv>iii>i>iiBii>i>iii>ivCi>ii>iv>iiiDiii>ii>i>iv
Aiv>iii>i>iiBii>i>iii>ivCi>ii>iv>iiiDiii>ii>i>iv
Arrange the following alcohols in order of increasing reactivity towards Lucas reagent: 2-butanol, 1-butanol, 2-methyl-2-propanol.
View SolutionArrange the following metals in the decreasing order of chemical reactivity :
Cu, Mg, Fe, Ca, Zn, Na.
View SolutionArrange the following compounds in order of decreasing reactivity towards SN1 reaction
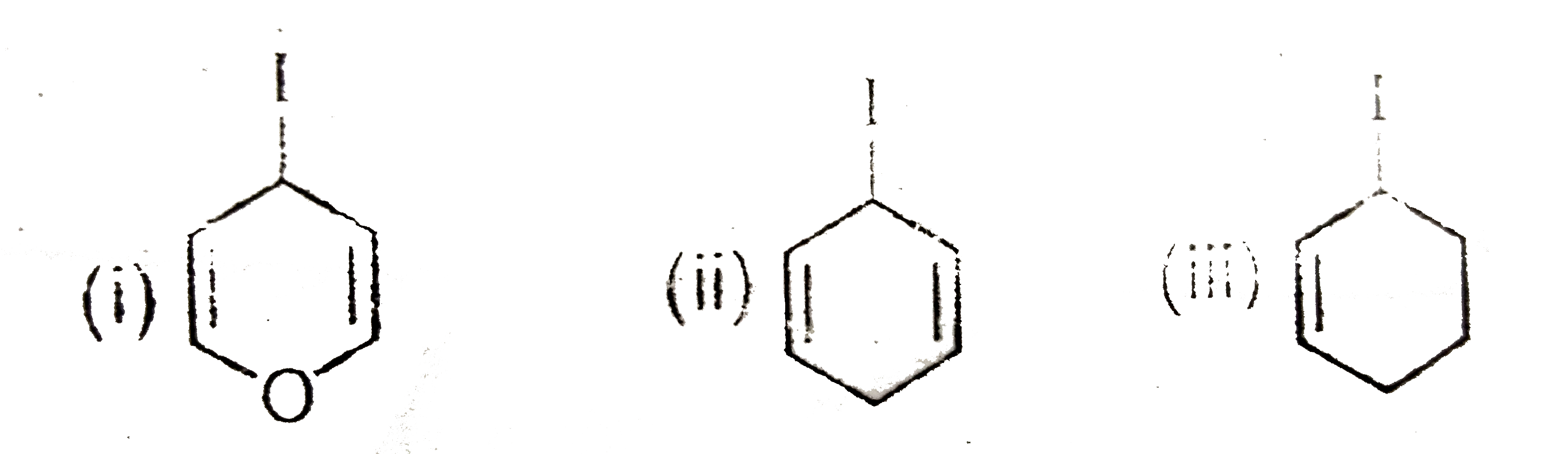
View SolutionArrange the following metal in the increasing order of their reactivity towards water, Zinc, Iron, Magnesium, Sodium
View SolutionArrange the following three chortides in decreasing order toward SN1 reactivity.
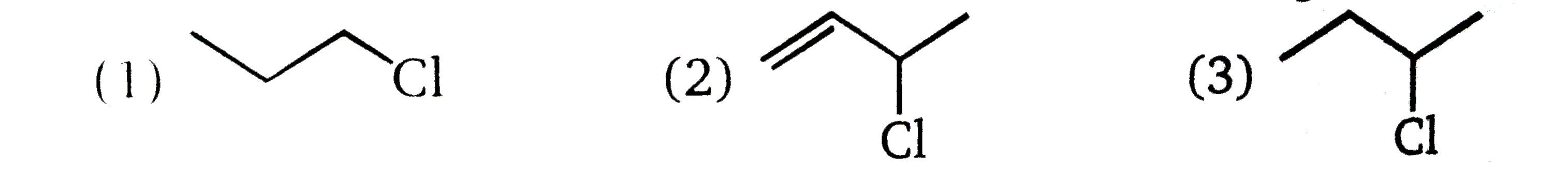
View Solution

