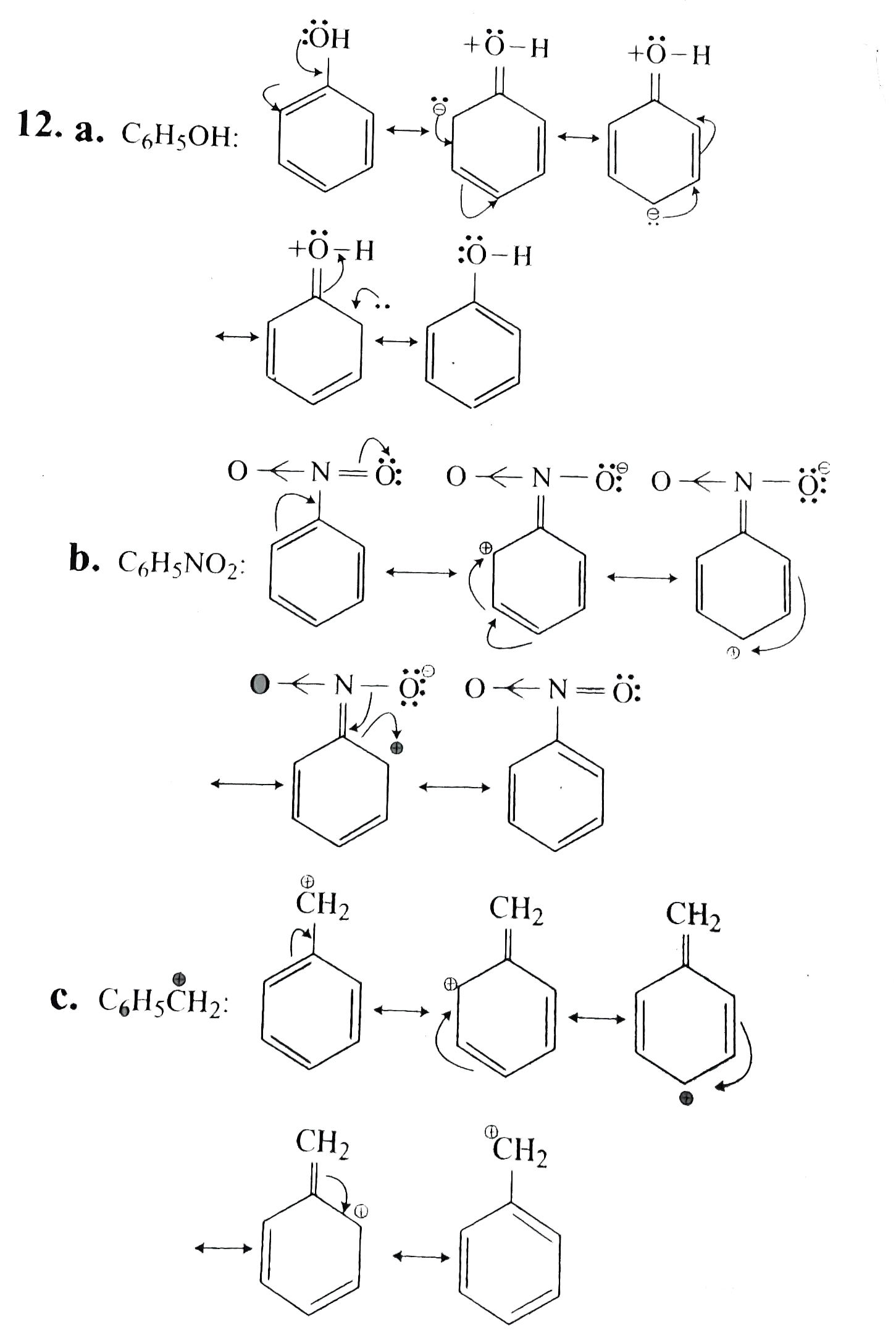
Draw the resonance structures for the following compounds. Show the electron shift using curved-arrow notation.
(a) C6H5OH
(b) C6H5NO2
( c) C6H5⊕CH2
(d) CH3CH=CHCHO
( e) CH3CH=CH−⊕CH2
(f) C6H5CHO
(g) CH2=CHOCH3.
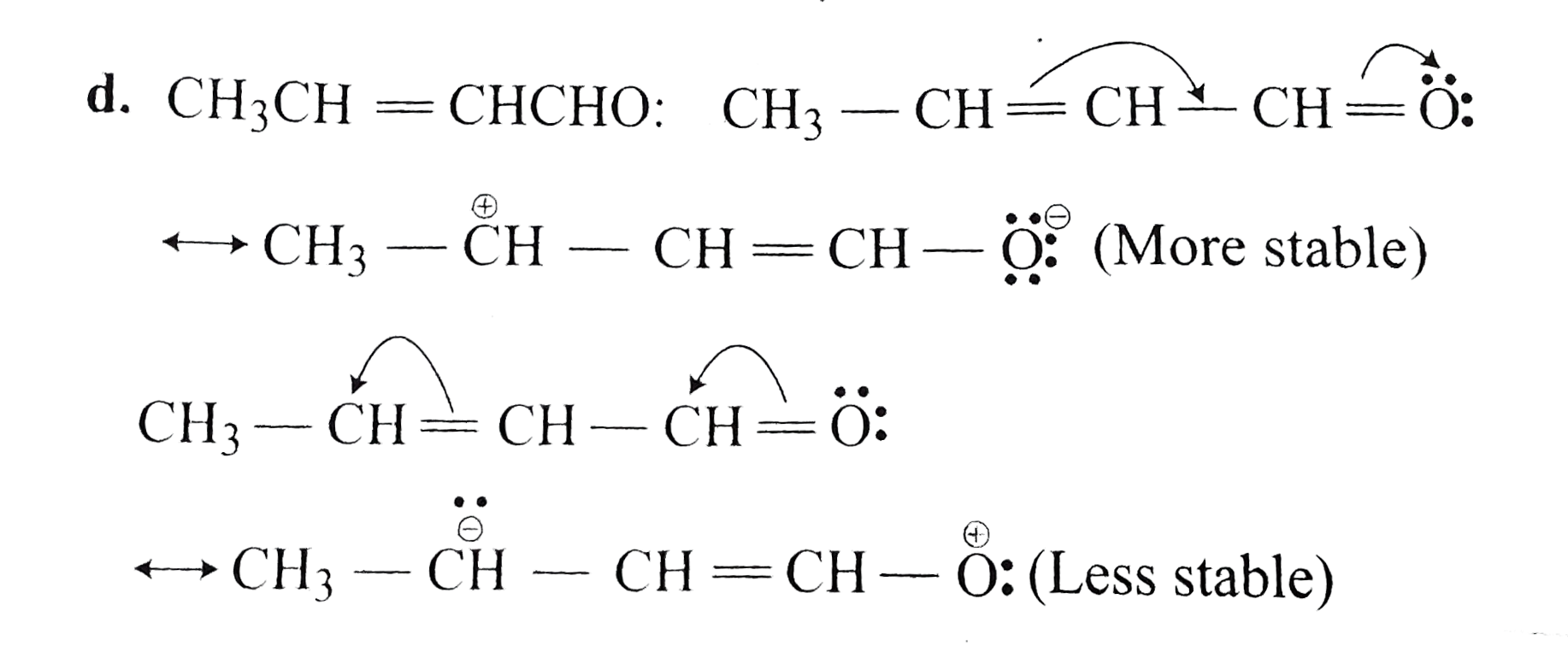 .
.
(Electropositive element with positive cahrgeElectronegative element with negative charge)
Similar Questions
Knowledge Check
The reaction
C6H5CHO+CH3CHOdil.NaOH−−−−−→
C6H5CH = CHCHO is calledABenzoin condensationBClaisen condensationCPerkin reactionDCannizzaro's reactionC6H5−CH=CHCHO(X)−−→C6H5CH=CH2OH
In the above sequence (X) can be:AH2/NiBNaBH4CK2Cr2O7/H+DBoth a and bC6H5CH=CHCHOX−−→C6H5CH=CHCH2OH
In the above sequence X can be :AH2/NiBNaBH4CK2Cr2O7/H+DBoth (a) and (b)
Draw the resonance structures for the following compounds. Show the electron shift using curved-arrow notation.
CH3CH=CH+CH2
View SolutionDraw the resonance structures for the following compounds. Show the electron shift using curved-arrow notation.
(a) C6H5OH (b) C6H5NO2 (c) CH3CH=CHCHO (d) C6H5–CHO (e) C6H5−+CH2 (f) CH3CH=CH+CH2
View SolutionDraw the resonance structures for the following compounds. Show the electron shift using curved-arrow notation.
(a) C6H5OH (b) C6H5NO2 (c) CH3CH=CHCHO (d) C6H5–CHO (e) C6H5−+CH2 (f) CH3CH=CH+CH2
View SolutionClassify the reagents shown in bonds in the following equations as nucleophilies or electrophiles. Use curved-arrow notation to show the electron movement.
(a) CH_3COOH + HO^(Ө) rarr CH_3COO^(Ө) + H_2 O
(b) CH3COCH3+NCӨ→CH3C(CN)OHCH3
( c) C6H6+CH3⊕CO→C6H5COCH3.
View SolutionThe stablest radical among the following is
(a) C6H5CH2−⋅CH2
(b) CH3C⋅H2
(c) C6H5−⋅CH−CH3
(d) CH3−⋅CH−CH3
(e) CH3−CH2−C⋅H2
View Solution

