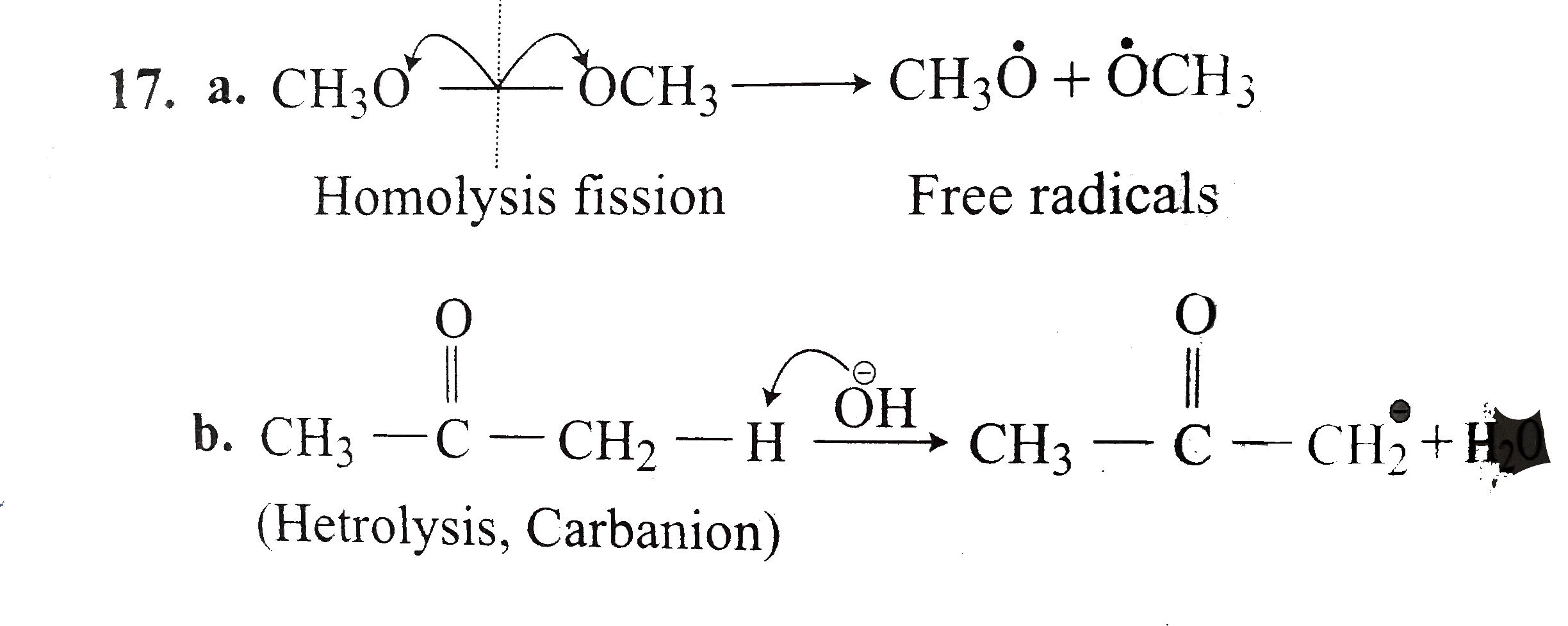
For the following bond cleavages, use curved-arrow to show the electron flow and classify each as homolysis or heterolysis. Identify intermediate products as free radical, carbocation, and carbanion.
(a) CH3O−OCH3→CH3.O+.OCH3
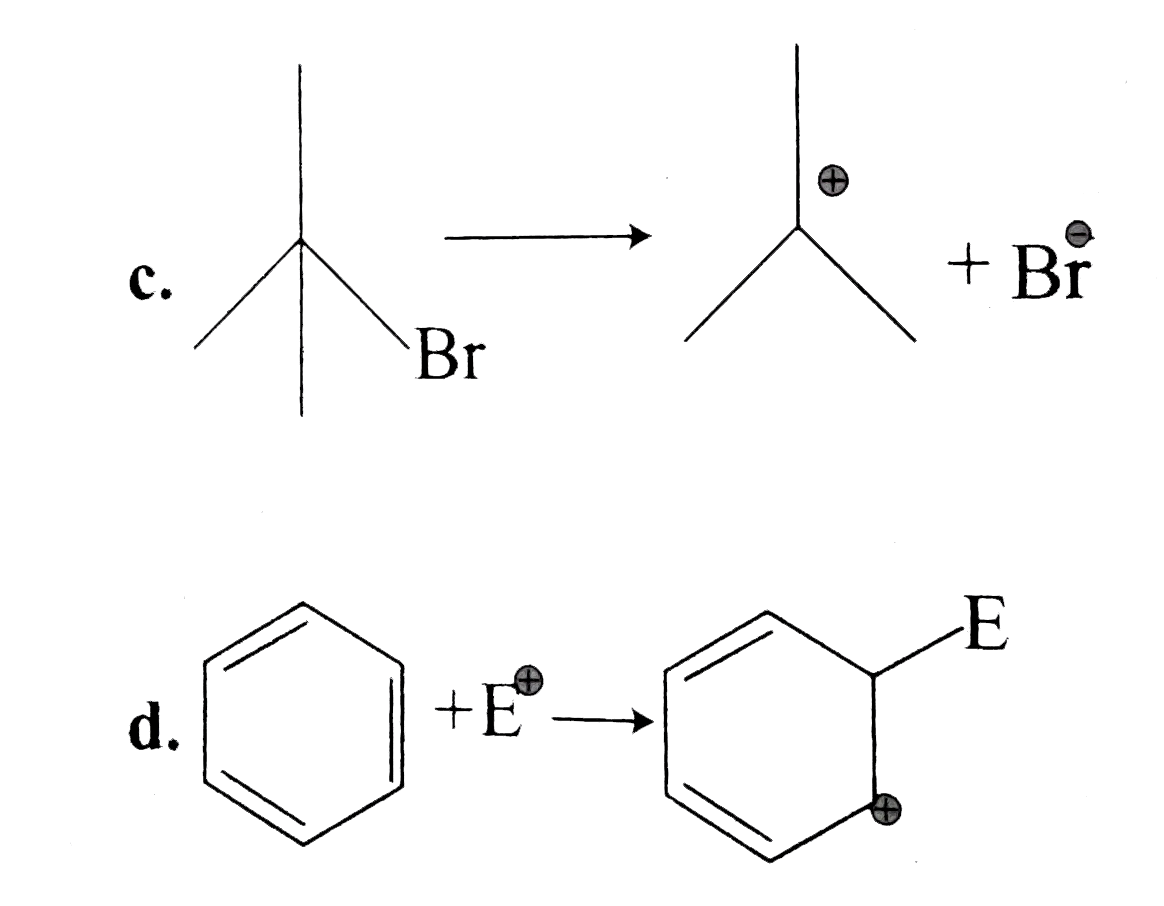 .
.
Similar Questions
For the following bond cleavages, use curved arrows to show the electron flow and classify each as homolysis or heterolysis. Identify reactive intermediate produced during the fission.
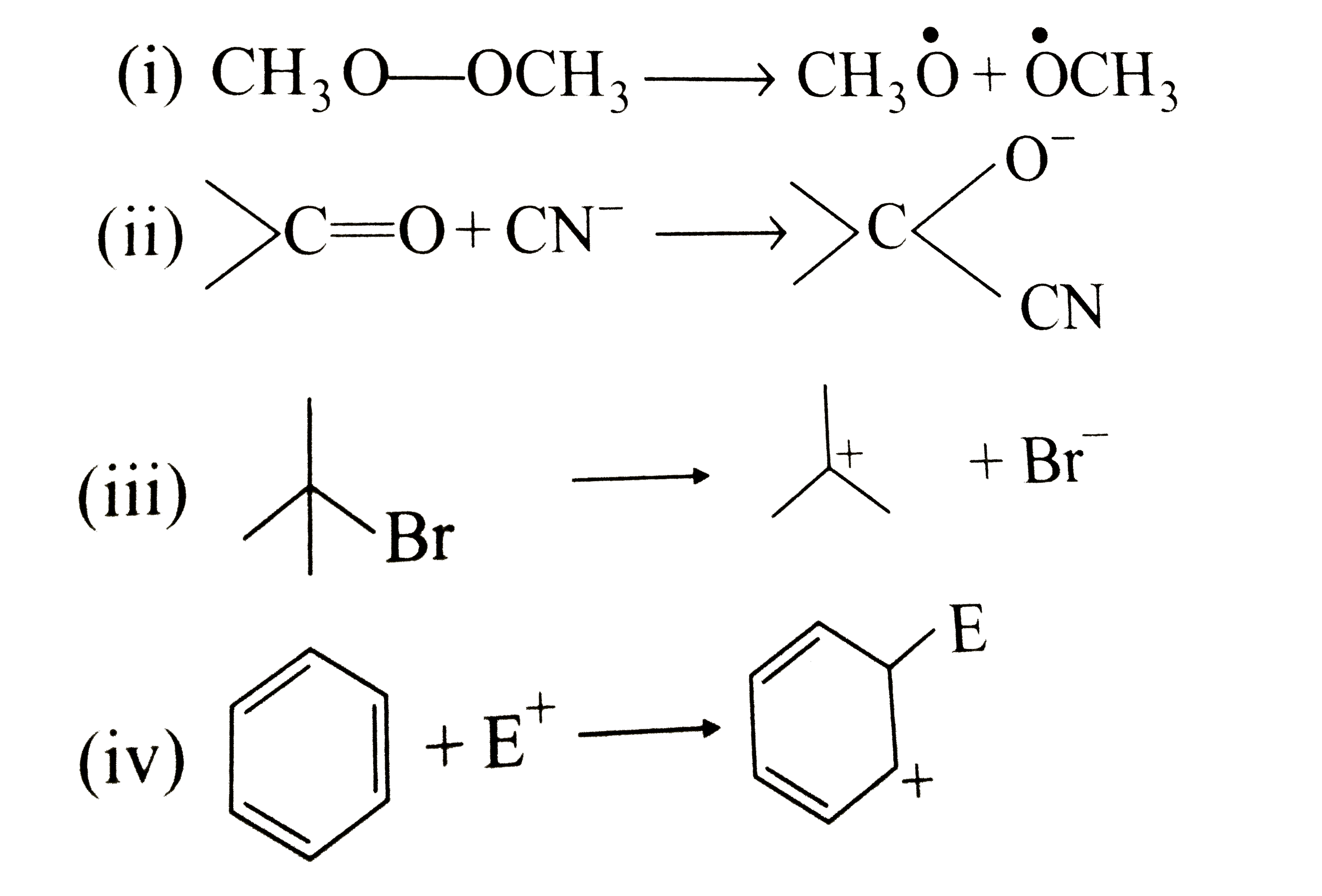
View SolutionClassify each of the following as homolysis as homolysis or heterolysis. Identify the reaction intermediates produced , as free radical, carbocation and carbanion.
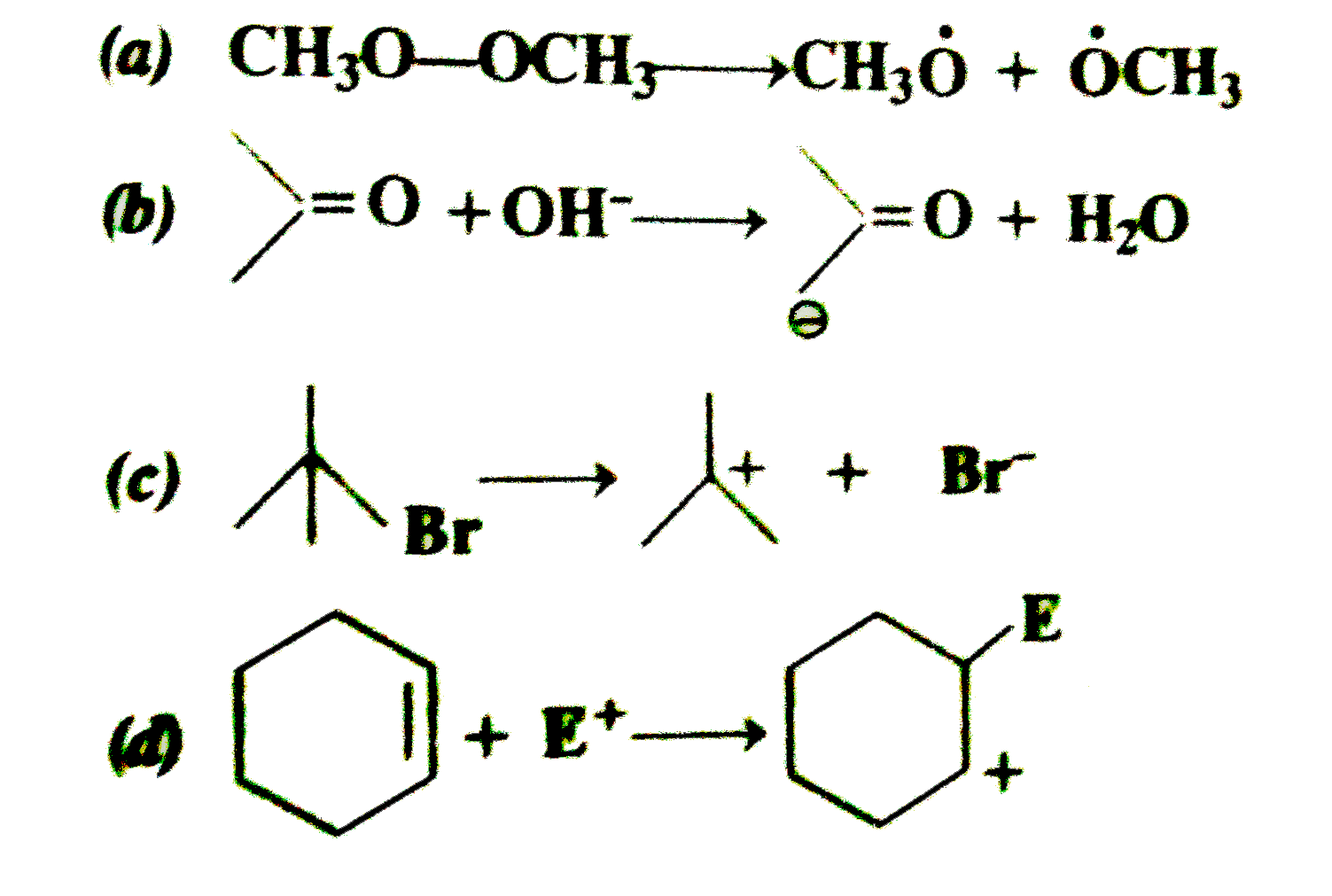
View Solution
Knowledge Check
CH3O−CH3 is
ALewis acidBArrhenius acidCLewis baseDBronsted acidCH3−O−CH3 is:
ALewis acidBArrhenius acidCLewis baseDBronsted acid
Identify the type of the following reaction of carbon compounds.
CH3−CH2−CH3→3CO2+4H2O
View SolutionCH3−CH2−O−CH3
View SolutionUsing the curved-arrow notation, show the formation of reactive intermediates when the following convalent bonds undergo heterolytic cleavage :
(a) CH3−S−CH3
(b) CH3−CN
( c) CH3−Cu .
View SolutionIdentify the type of the following reaction of carbon compounds.
CH3−CH2−CH3+5O2→3CO2+4H2O
View SolutionIdentify the type of the following reaction of carbon compounds.
CH3−CH2−CH3+5O2→3CO2+4H2O
View SolutionUsing the curved-arrow notation, show the formation of reactive intermediates when the following convalent bonds undergo heterolytic cleavage :
(a) CH3−S−CH3
(b) CH3−CN
( c) CH3−Cu .
View Solution