The decreasing nucleophilicity of the following is :
(I) CH3SӨ
(II) CH3OӨ
(III) ӨOH
(IV) EtOӨ.
IV>III>II>I
I>II>III>IV
IV>III>I>II
II>I>III>IV
The correct Answer is:C
( c) Acidity as explained in (a) is : CH2SH>CH3OH>H2O>EtOH
Basicity : CH3SӨ<CH3ӨO<ӨOH<EtӨ
When nucleophilic centre is different (CH3SӨ and CH3ӨO) and they belong to the same group, nucleophilecity antiparallels basicity.
∴ nucleophilicity : EtӨO>ӨOH>CH3ӨS>CH3ӨO, so the answer is c.
Similar Questions
The decreasing order of +I effect of the following is :
(I) −OӨ
(II) Me−
(III) Et−
(IV) Me2CH−
(V) Me3C−.
View SolutionOf the following pairs, which one is the stronger base and stronger nucleophile ?
(a) (I) MeOӨ, (II) MeOH
(b) (III) ӨOH, (IV) ӨCN
(c) (V) H2O, (VI) ӨOH
(d) (VII) ӨNH2, (VIII) NH3.
View Solution
Knowledge Check
The decreasing order of nucleophilicities of the following is :
(I) H2O
(II) EtOH
(III) MeCOOӨ
(IV) ӨOH
(V) EtOӨ.A(V)>(IV)>(III)>(II)>(I)B(I)>(II)>(III)>(IV)>(V)C(IV)>(V)>(III)>(II)>(I)D(I)>(II)>(III)>(V)>(IV)The decreasing basic character of the following is :
(I) PhOӨ
(II) CH3SӨ
(III) ӨOH
(IV) MeOӨ.AI>II>III>IVBIII>IV>II>ICIV>III>II>IDI>II>IV>IIIThe decreasing order of nucleophilicities of the following is :
(I) BrӨ
(II) MeOӨ
(III) Me2NӨ
(IV) Me3CӨ.A(I)>(II)>(III)>(IV)B(IV)>(III)>(II)>(I)C(IV)>(III)>(I)>(II)D(II)>(I)>(III)>(IV)
Compare the stability of the following
(a)ӨCH3 (b)ӨNH2 (c)ӨOH (d)ӨF
View SolutionThe decreasing order of the basic character of the following is :
(I) FӨ
(II) NOӨ3
(III) H2O
(IV) CH3OH.
View SolutionThe increasing order of pKb value of the following is :
(I) HC≡CӨ
HӨ
ӨNH2
(IV) CӨH3.
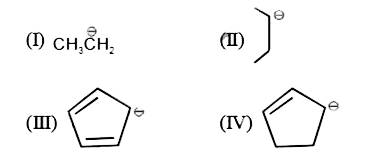
View SolutionThe order of stability of the following carbanion is
(I)CH3ӨCH2
View SolutionThe decreasing order of nucleophilicity among the nucleophiles is :
(I) CH3−C∣∣O−O−
(II)CH3O−
(III) NC− (IV)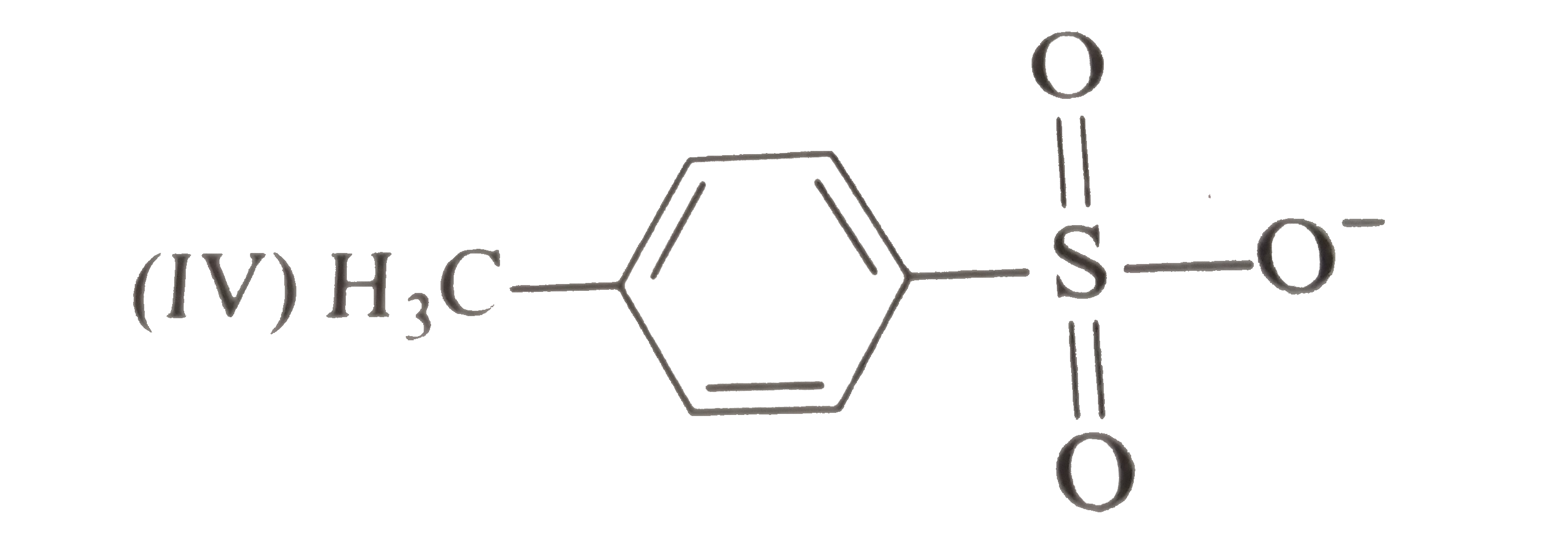
View Solution
