Explain the reaction :
(a) ROH+NaBr→
(b) ROH+HBr→
(c) 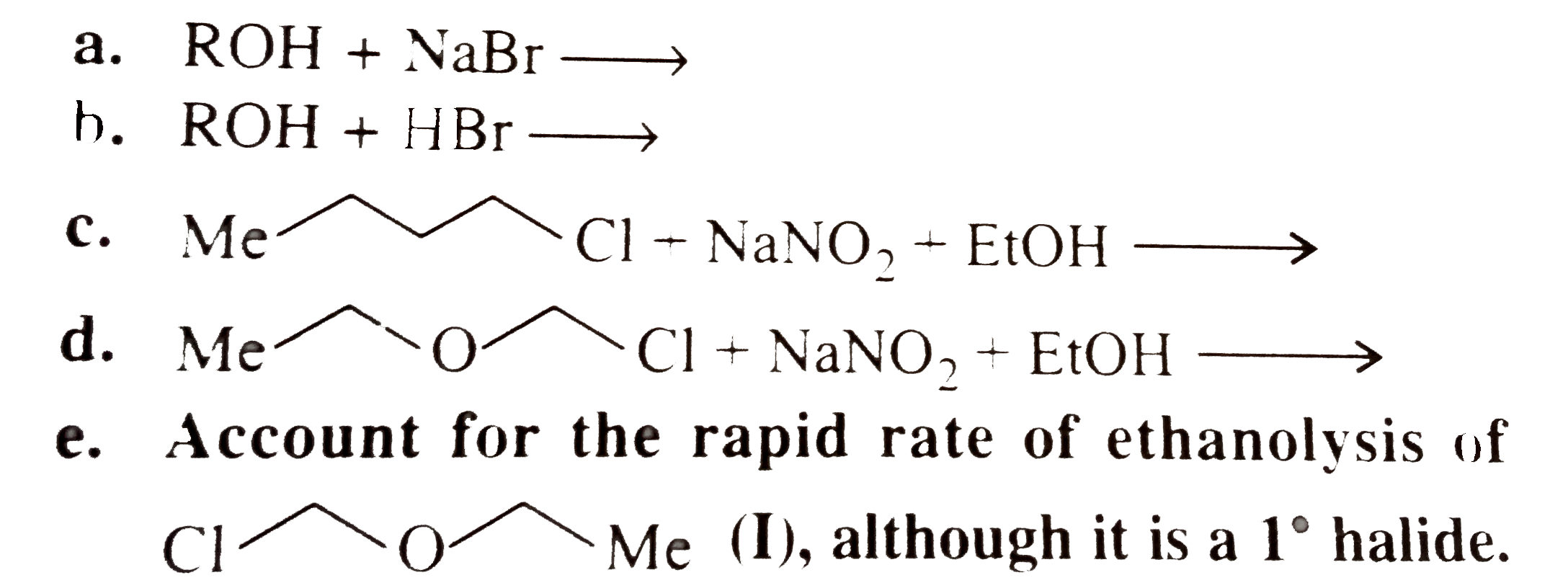
(a) No reaction. Extremely weak base Br^(Ө) cannot displace very strong base ӨOH.
(b) This reaction occurs in two steps.
ROH+HRr→ROH⊕2+BrӨ→RBr+H2O
Displacement is on the onium ion of ROH whose good leaving group property is due to the weekly basic H2O.
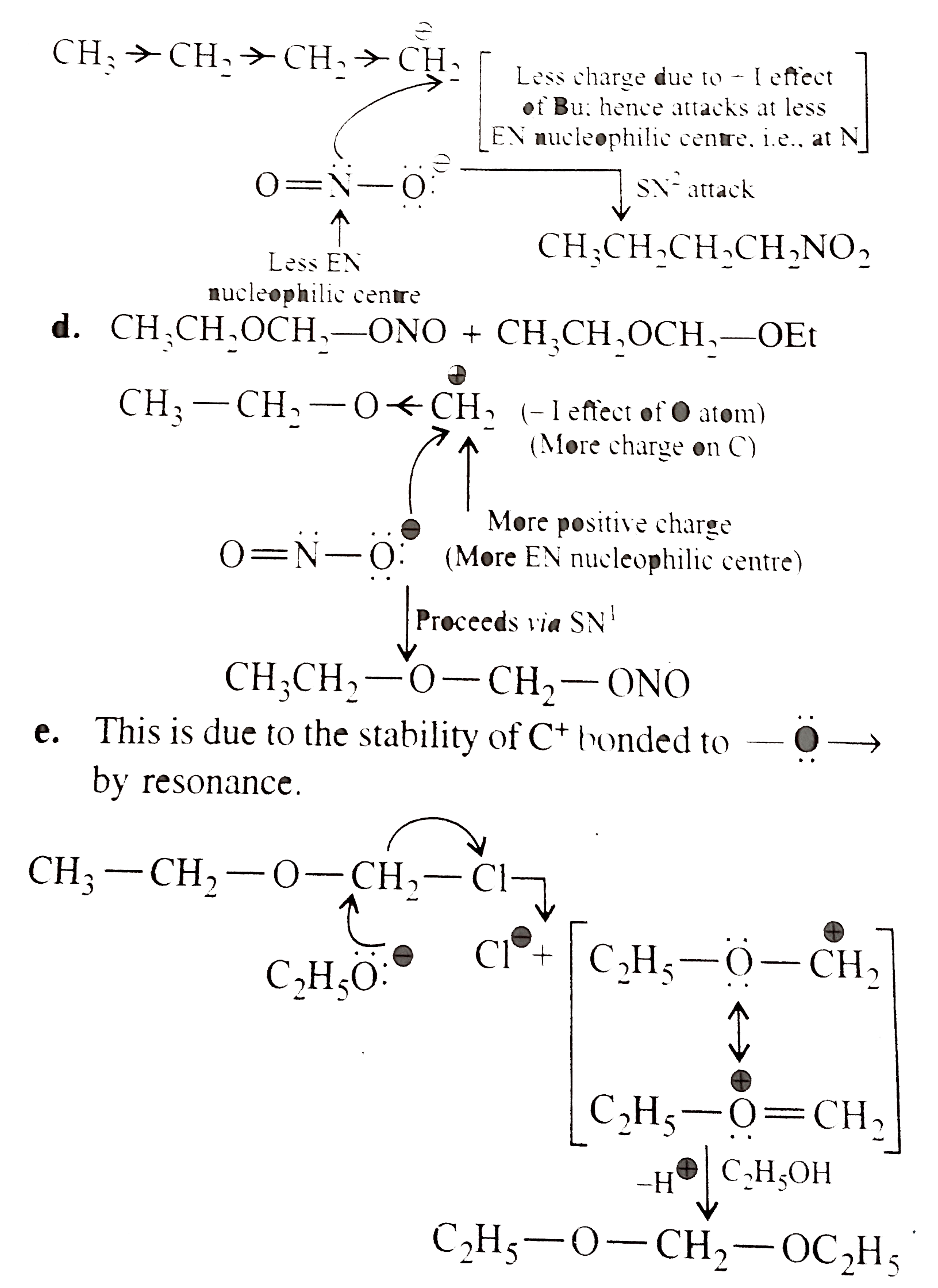
O∣∣N..−−..O..:(Ambident nucleophile)(Two nucleophilic centres N and O)Less ENnucleophiliccentreMore ENnucleophiliccentre[EN of O gt N]
Rule : If there is more positive charge on the carbonium formed, it will attack at the more EN nucleophilic centre and vice versa.
Similar Questions
Knowledge Check
The rate law for the reaction, RCl + Na (a) → ROH + NaCl is given by, rate = K1 [RCl]. The rate of the reaction will be -
ADoubled on doubling the concentration of sodium hydroxideBHalved on reducing the concentration of alkyl halide to halfCDecreased on increasing the temperature of the reactionDUnaffected by increasing the temperature of the reactionThe reaction 2ROH+2Na→2RONa+H2 suggests that alcohols are
AbasicBamphotericCneutralDacidicThe rate law for the reaction
RCl+NaOH(aq)→ROH+NaCl is given by
Rate =k[RCl]. The rate of the reaction will beADoubled on doubling the concentration of sofium hydroxide.BHalved on reducing the concentration of alkyl halide to one half.CDecrease on increaisng the temperature of the reaction.DUnaffected by increaisng the temperature of the reaction.
Compare the following displacement reactions and account for any difference
(a) ROH+NaBr→
(b) ROH+HBr→
View SolutionThe rate law for the reaction
RCL+NaOH(aq)→ROH+NaCl is given by Rate =k[RCl]. The rate of the reaction will be
View SolutionAssertion: For the reaction RCl+NAOH(aq)→ROH+NACl, the rate of reaction is reduced to half on reducing the concentration of RCl to half.
Reason: The rate of the reaction is represented by k[RCl] i.e., it is a first-order reaction.
View SolutionAssertion (A): For the reaction
RCl+NaOH(g)→ROH+NaCl, the rate of reaction is reduced to half on reducing the cocentration of RCl to half.
Reason (R ): The rate of the reaction is represented by k[RCl], i.e., it is a first order reaction.
View SolutionThe rate law for the reaction
RCl+NaOH(aq)→ROH+NaCl is given by Rate =K1[RCl]. The rate of the reaction will be
View Solution

