Explain the following observation:
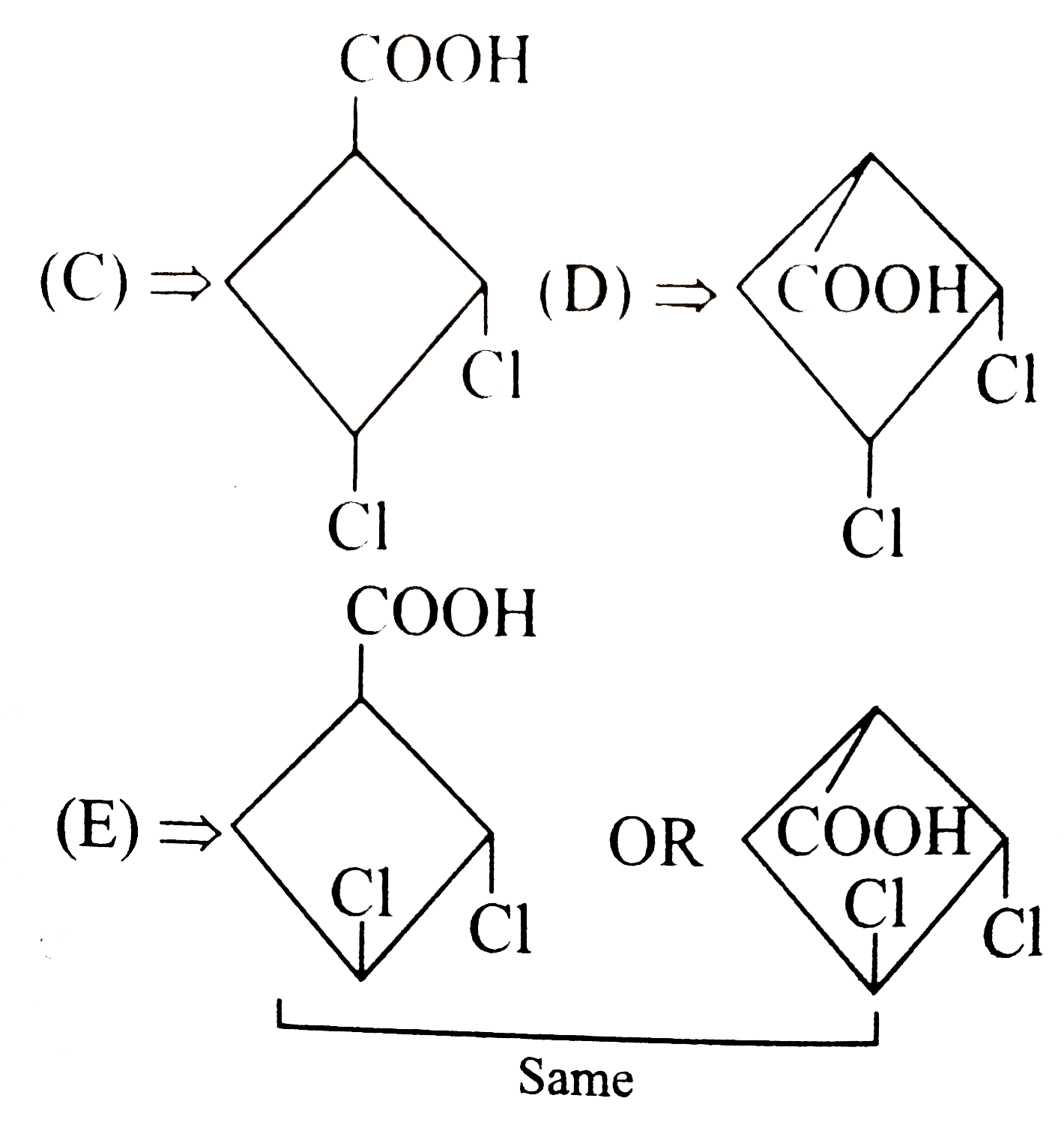
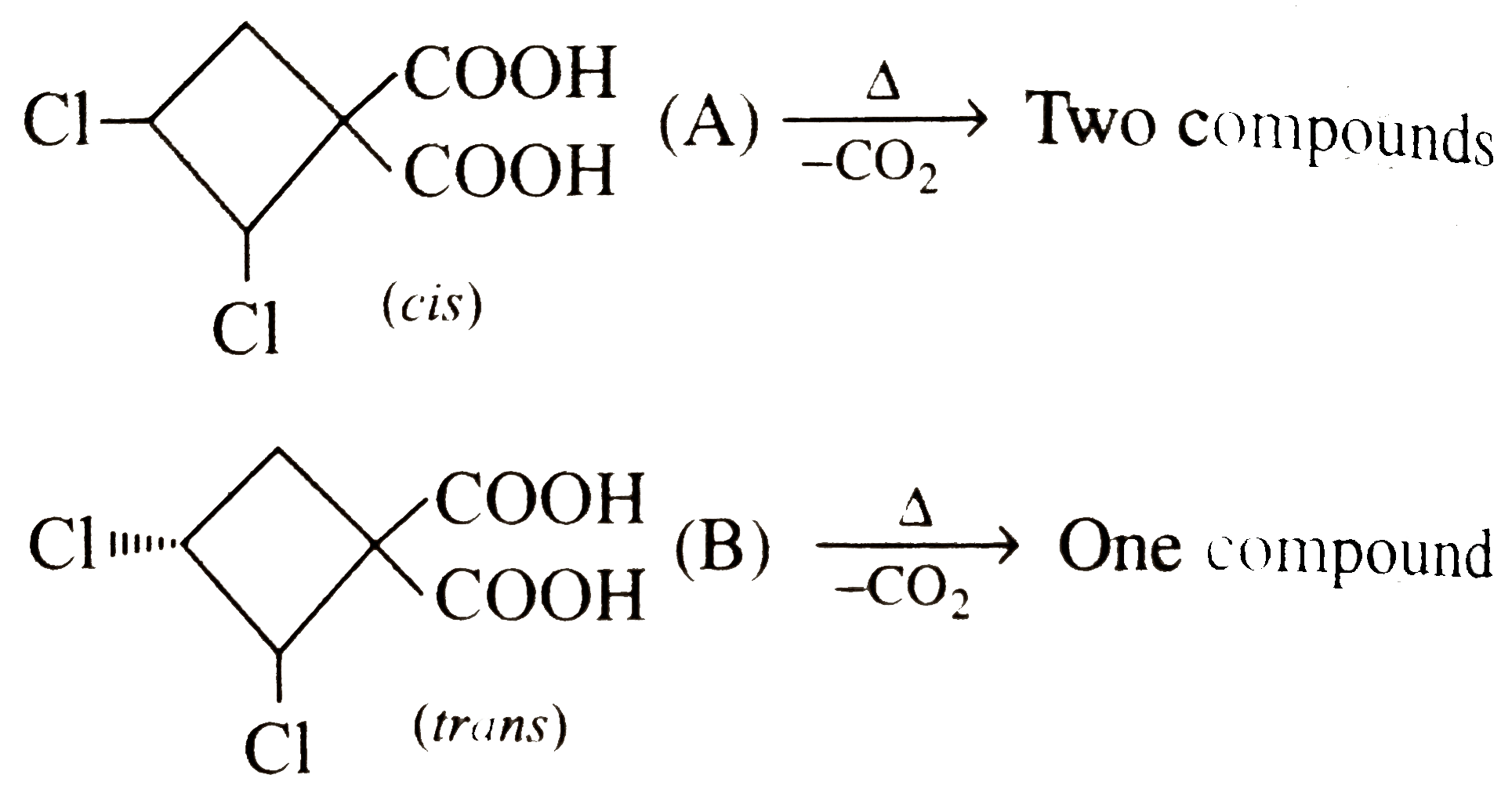 Two compounds (C and D)
Two compounds (C and D)
 One compound (E )
One compound (E )
Similar Questions
(a) On bolining a mineral (A) with Na2CO3 solution, a white precipirtate (B) is formed.
(b) The precipitate is fitered and the filtrate contains two conpounds (C) and (D). The componds (C) is removed by crystallisation and when CO2 is passed through the mother liquor left, (D) changes to (C).
( c) On strongly heating (C), two compounds (D) and (E) are formed.
(d) (E) on heating with cobalt oxide provides blue-coloured substance (F).
Identify (A) to (F) and give chemical reaction for (a) to (d).
View SolutionExplain the following observation:
(i) Transiton elements generally from colourd compounds.
(ii) Zinc is not regarded as a transition element.
View Solution
Knowledge Check
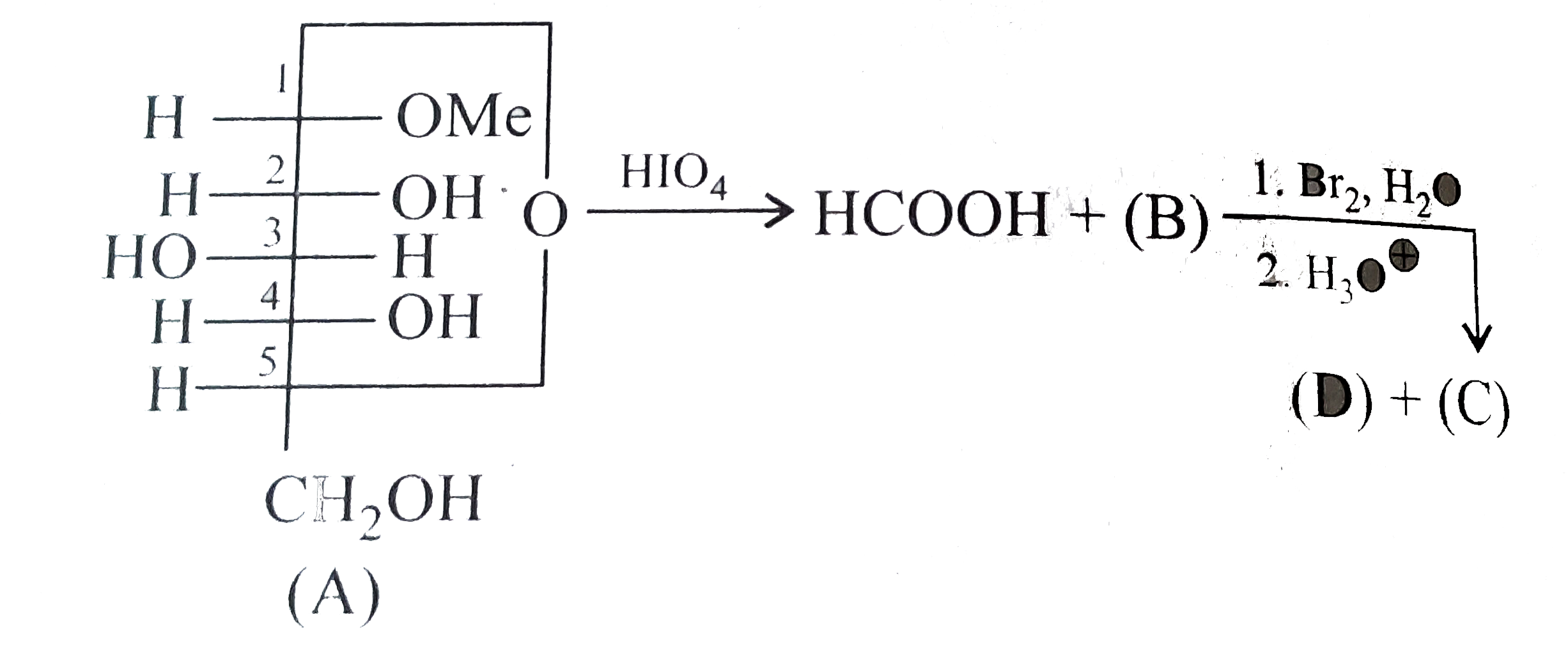
Compound (C) and (D) are:A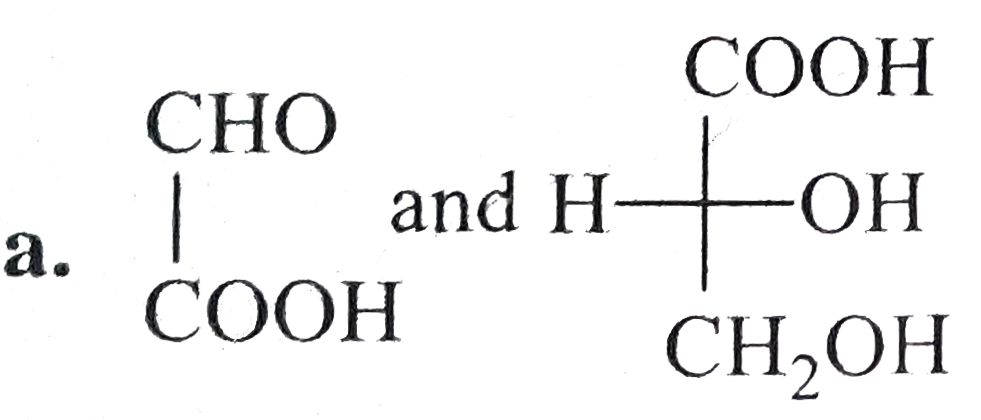 B
B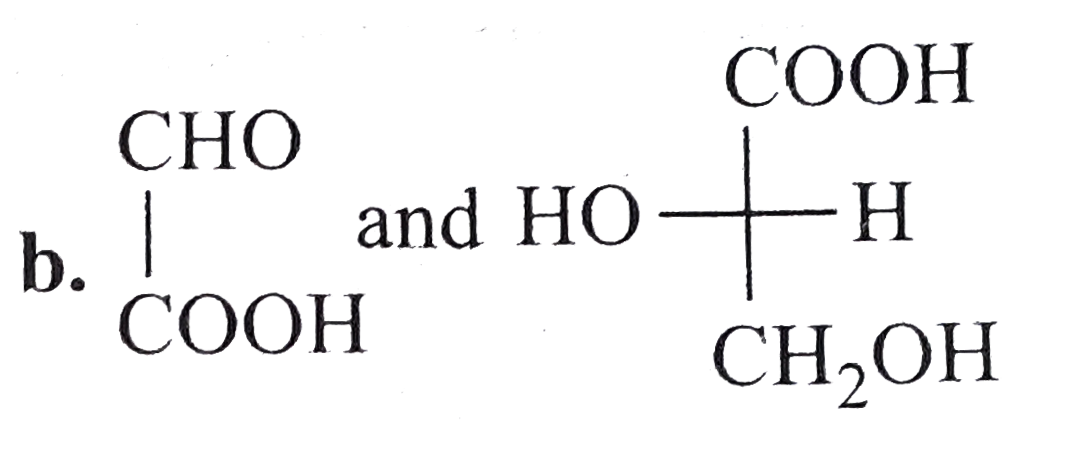 C
C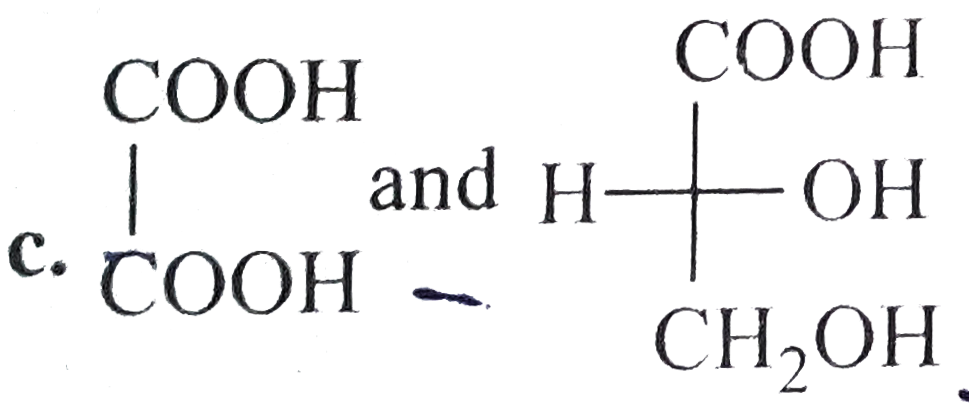 D
D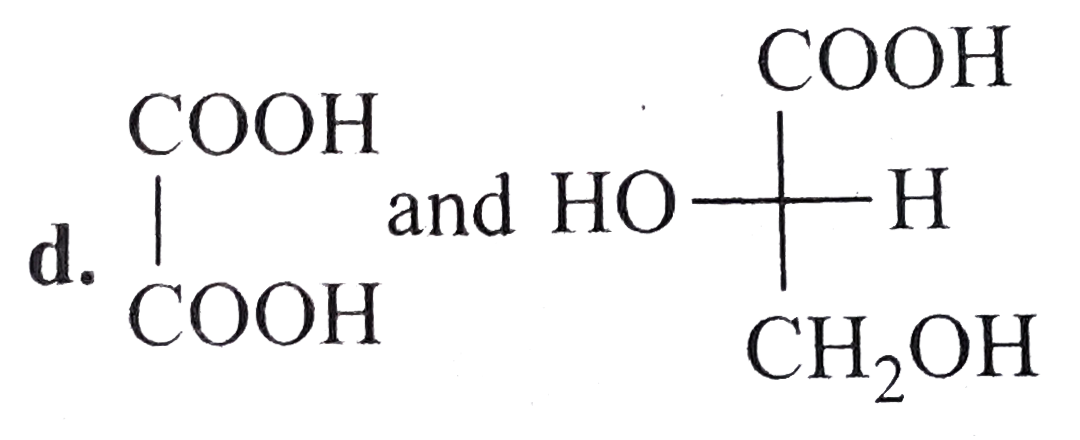
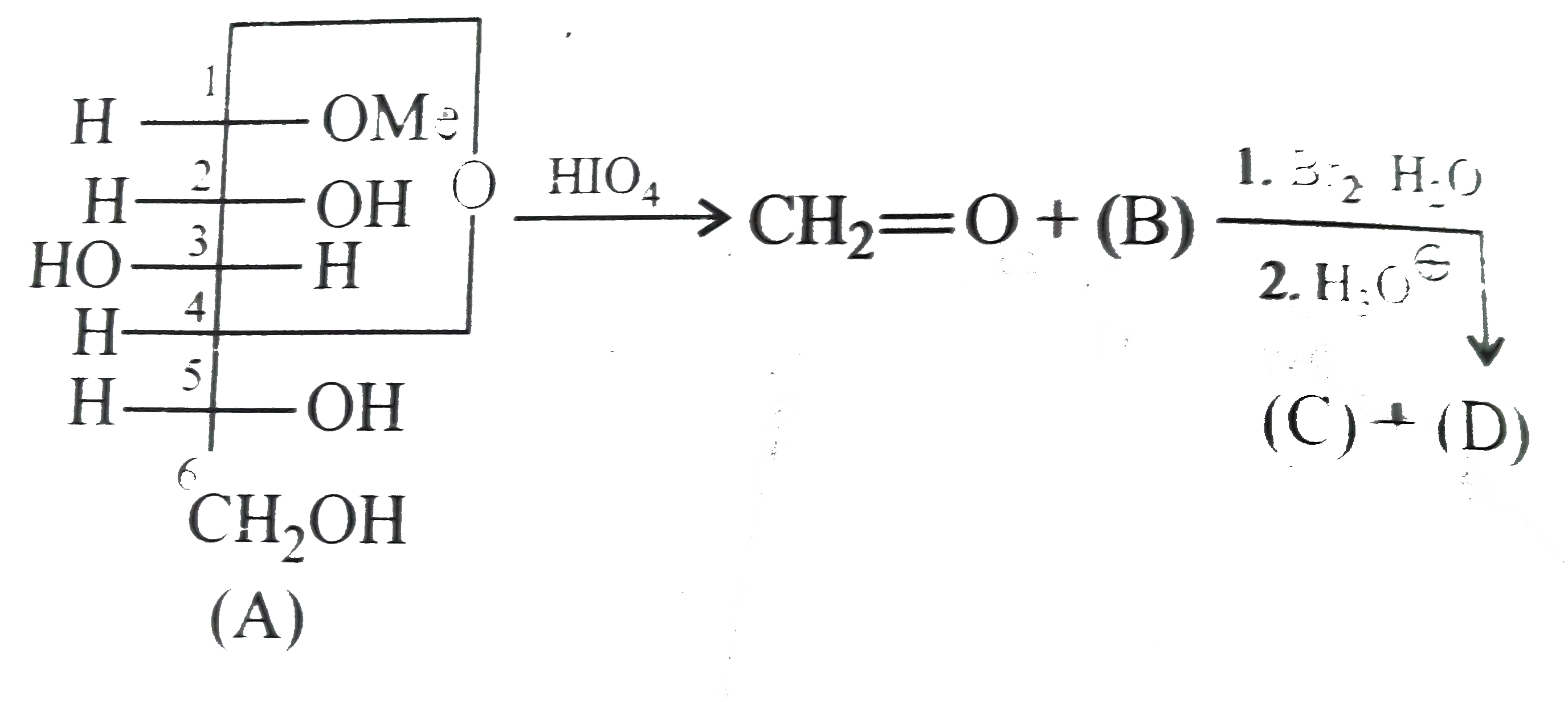
Compound (C) and (D) are:A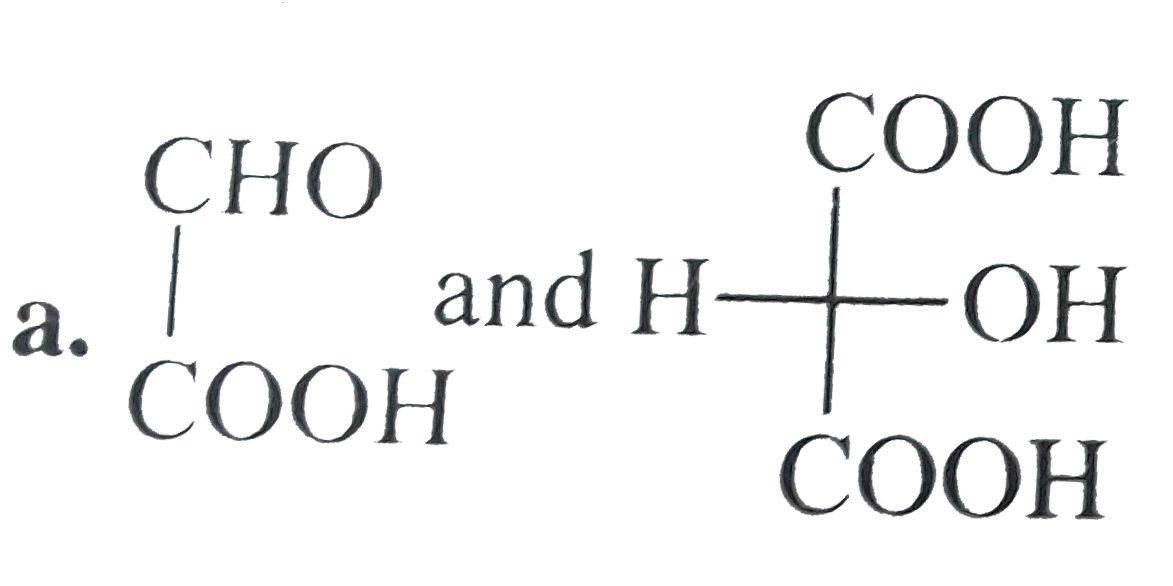 B
B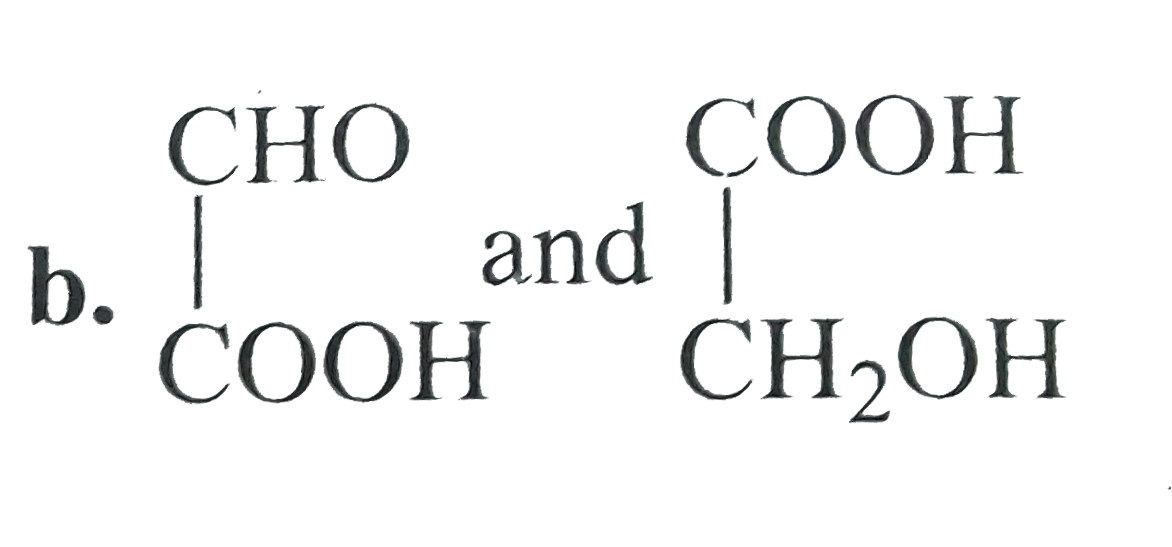 C
C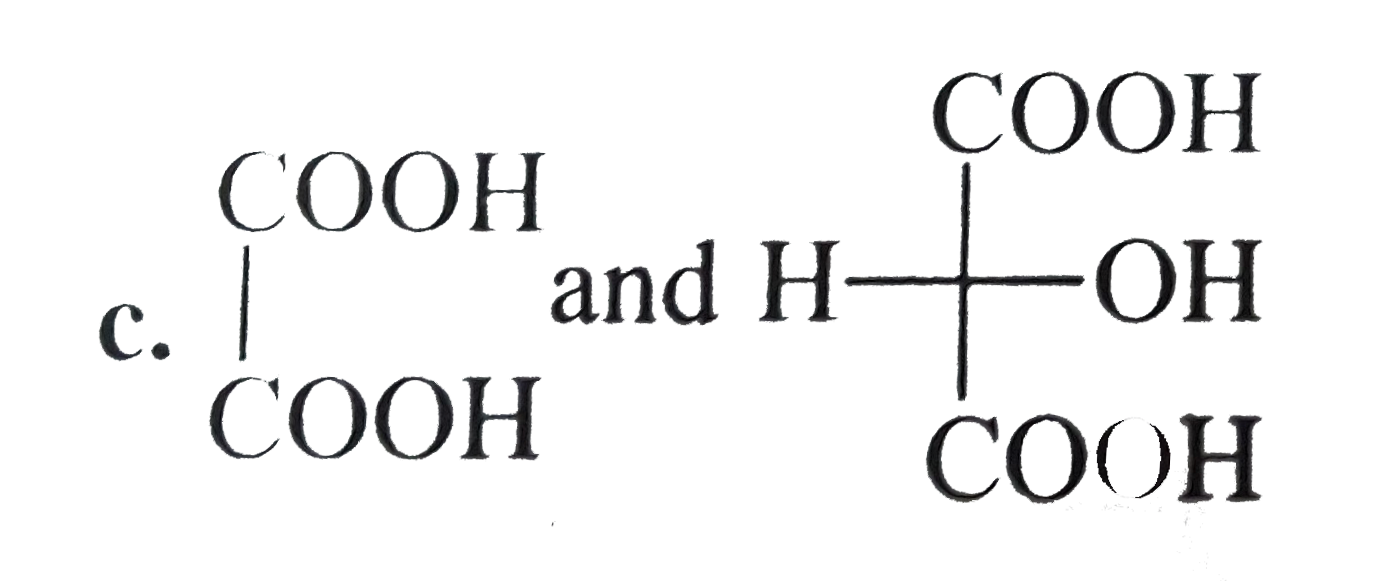 D
D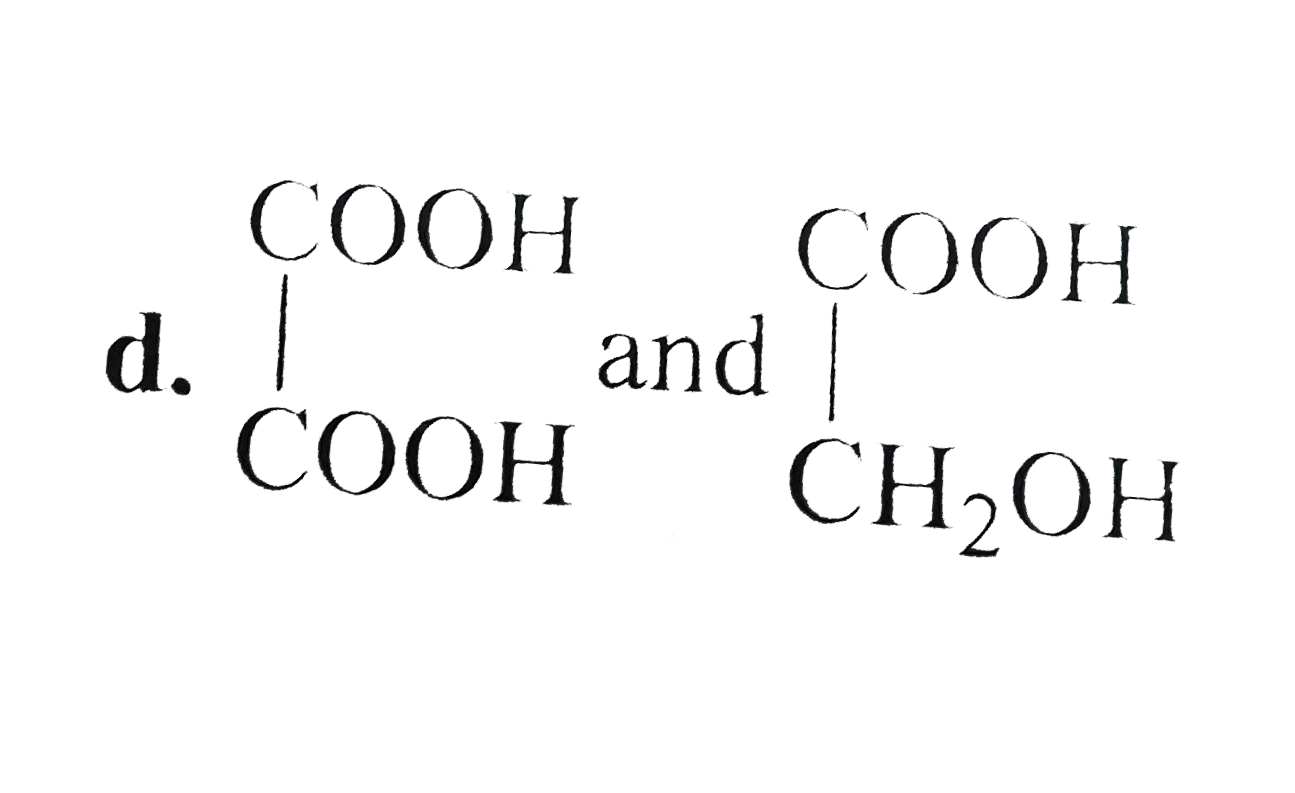
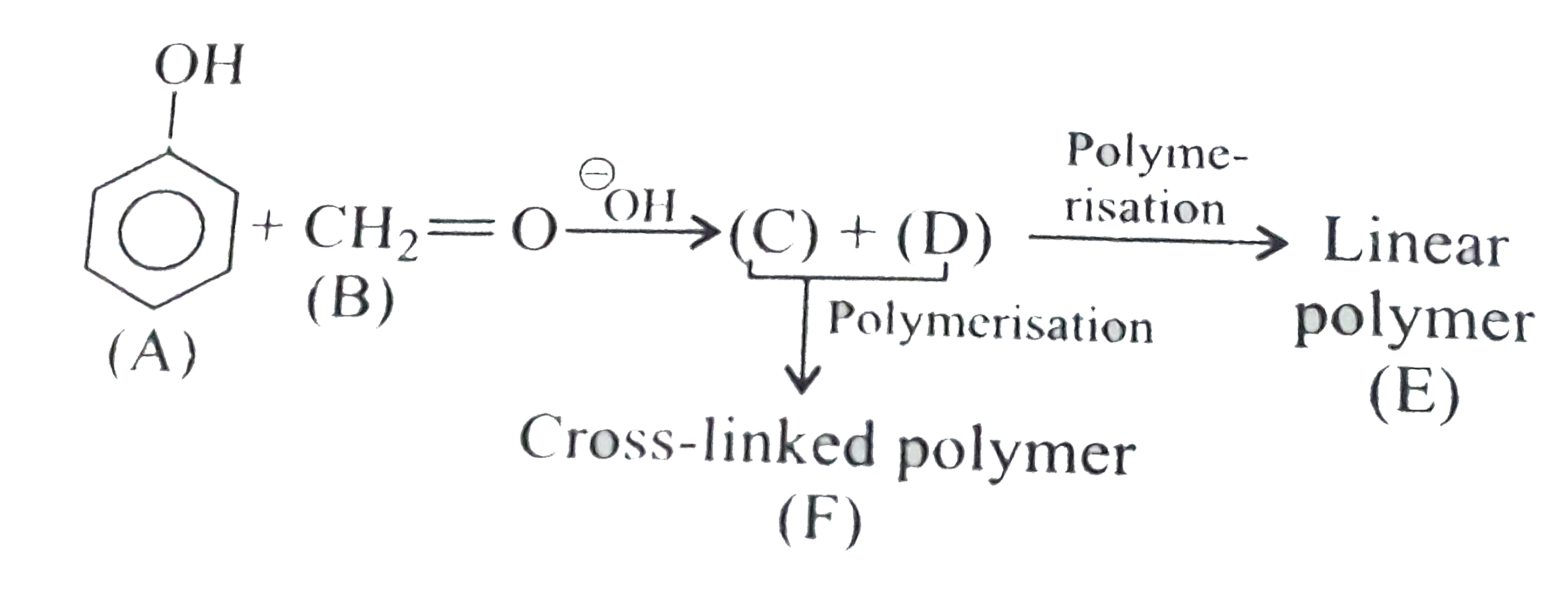
Compound (C ) and(D) are:A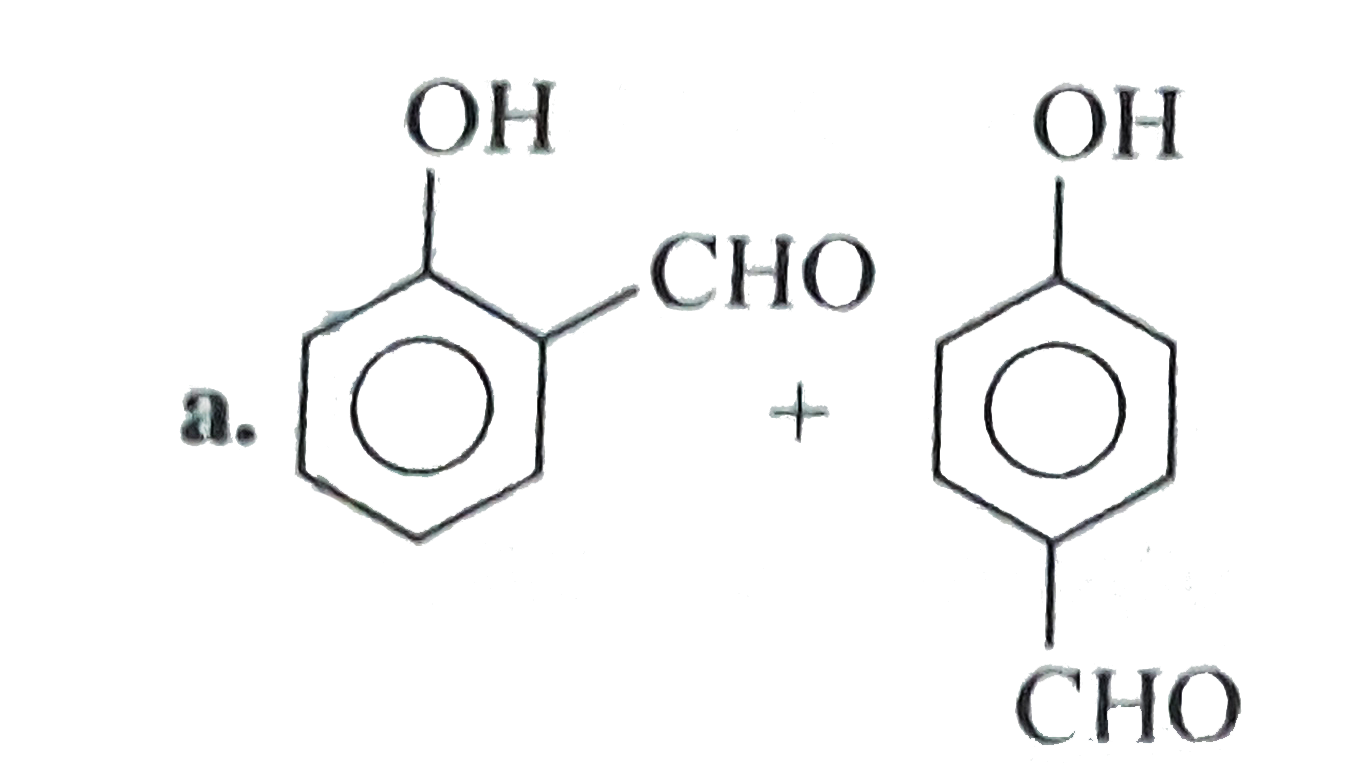 B
B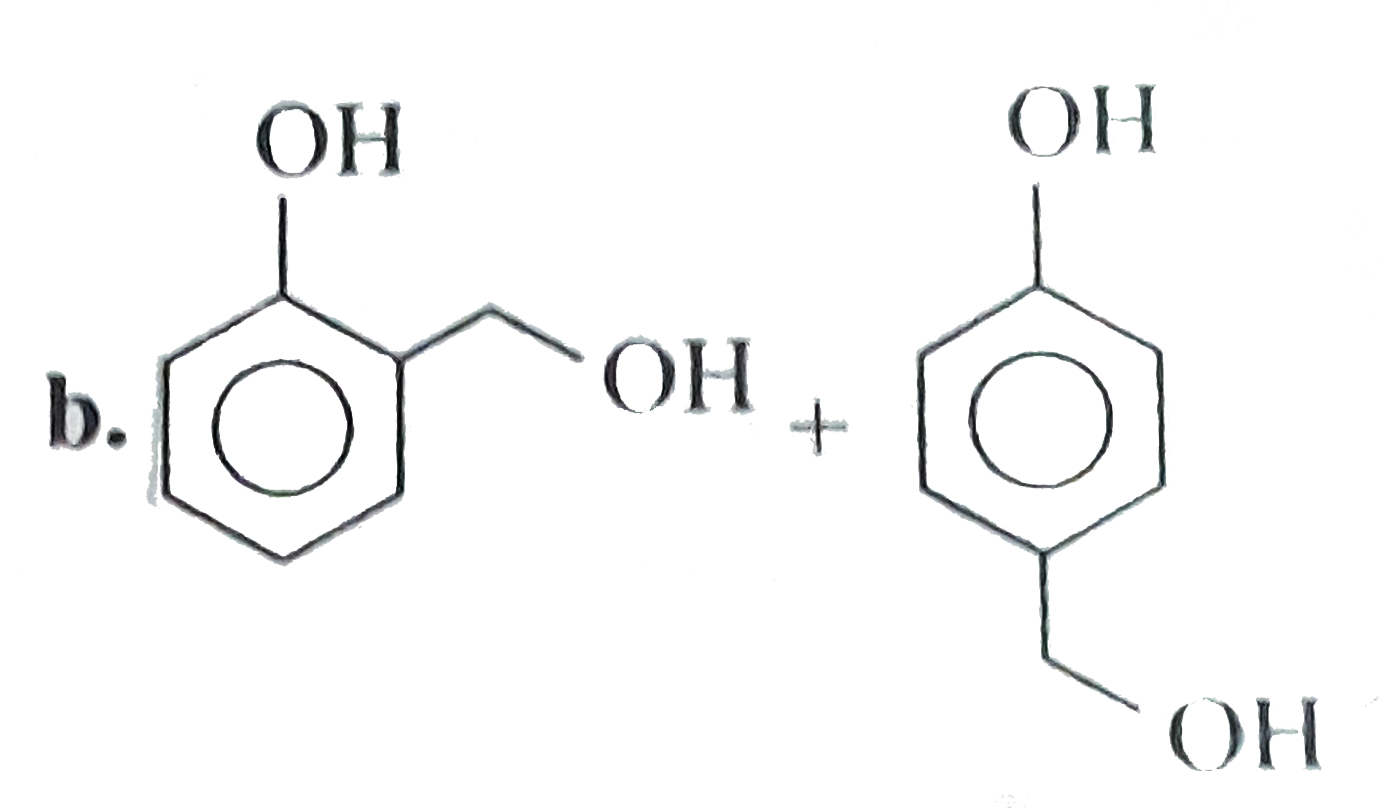 C
C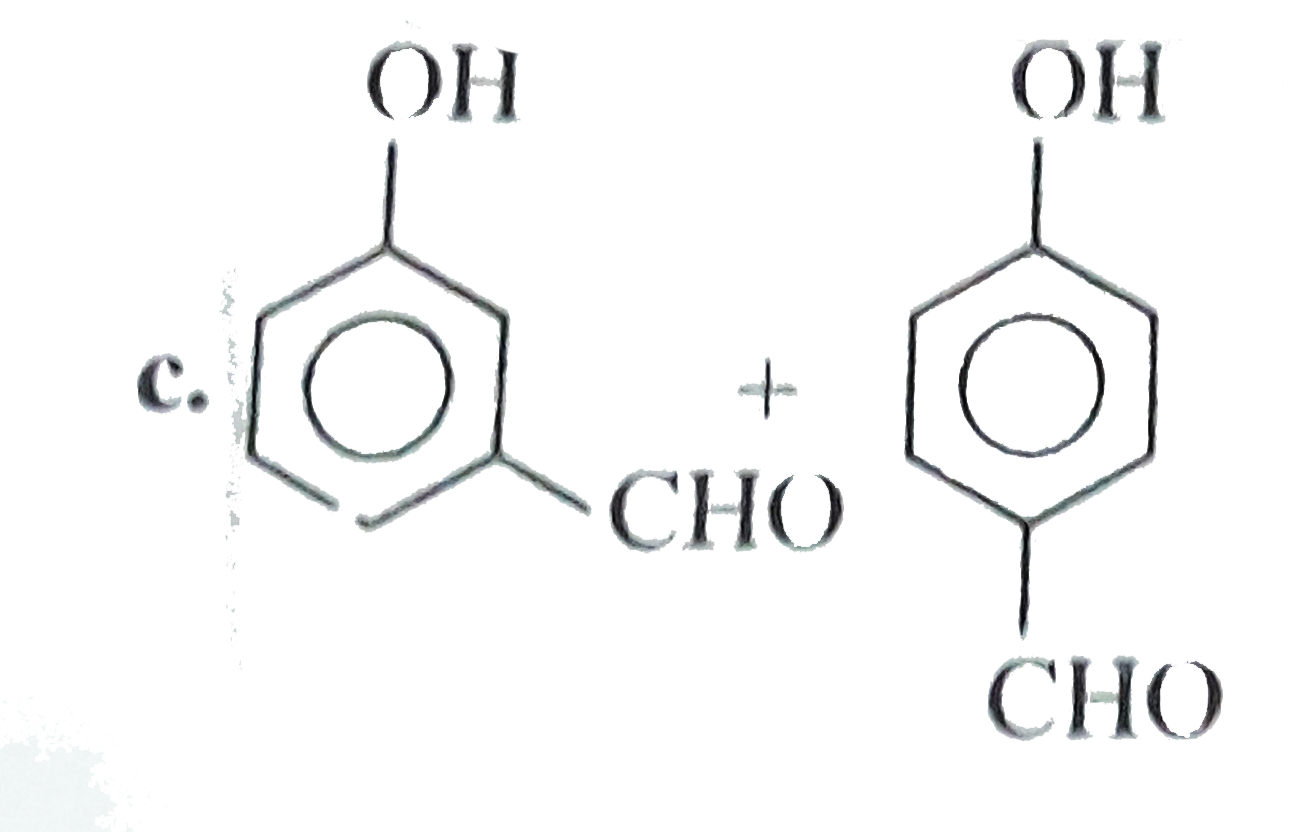 D
D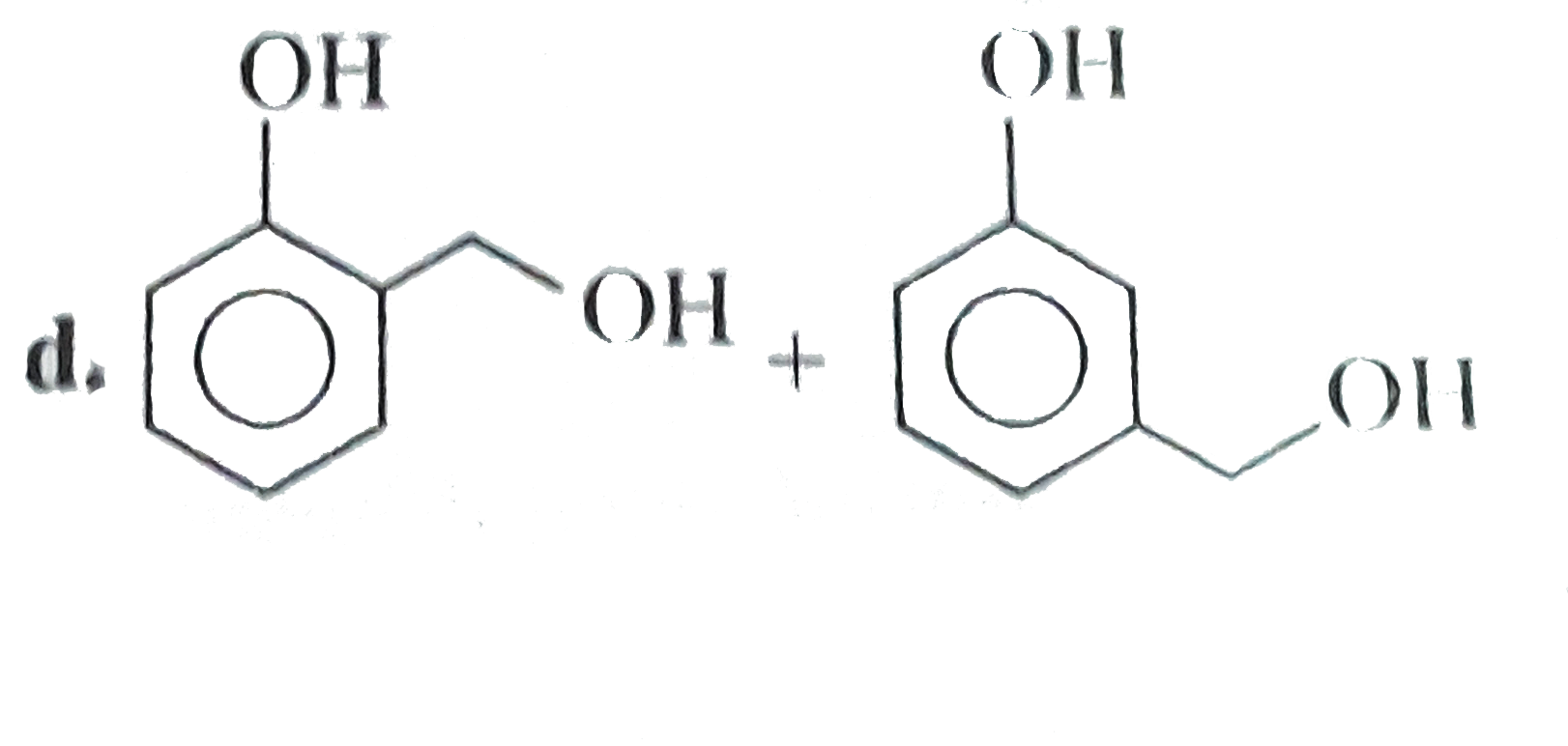
Explain
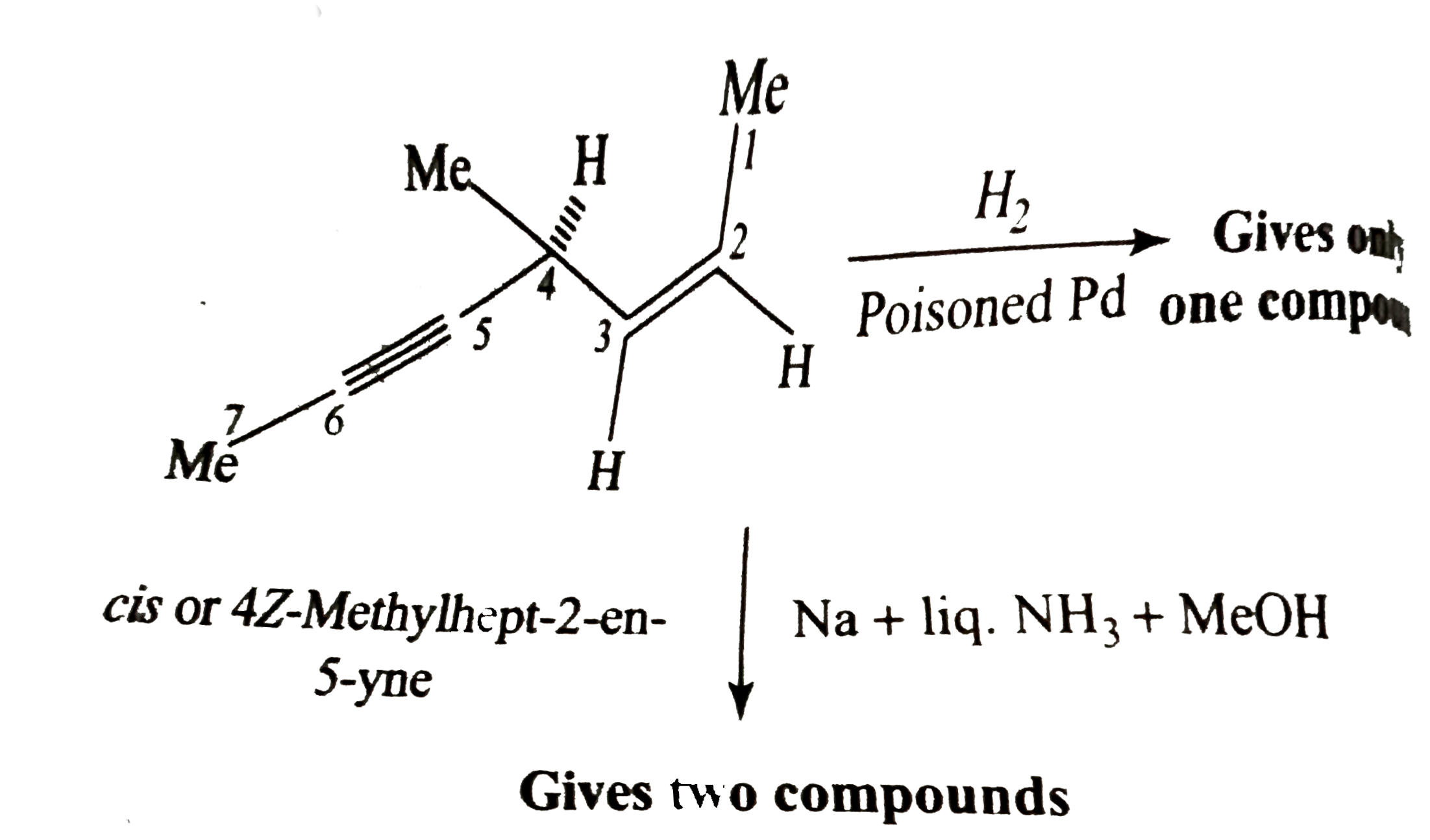
Gives two compounds.
View SolutionExplain the following observation :
(i) Transition elements generally form coloured compounds.
(ii) Zinc is not regaded as a transition element.
View Solution
The compounds (D) and (E ) are:
View Solution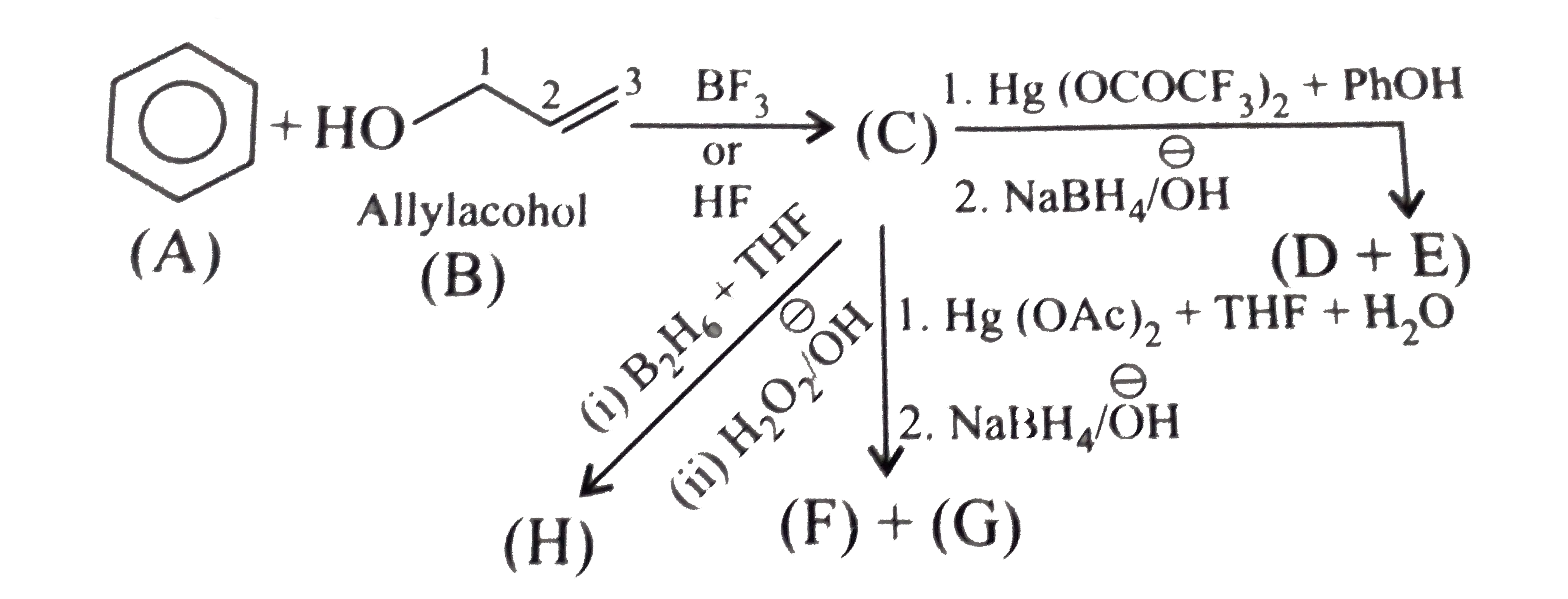
The compounds (D) and (E ) are:
View SolutionConsider the following reaction :
 ,
,
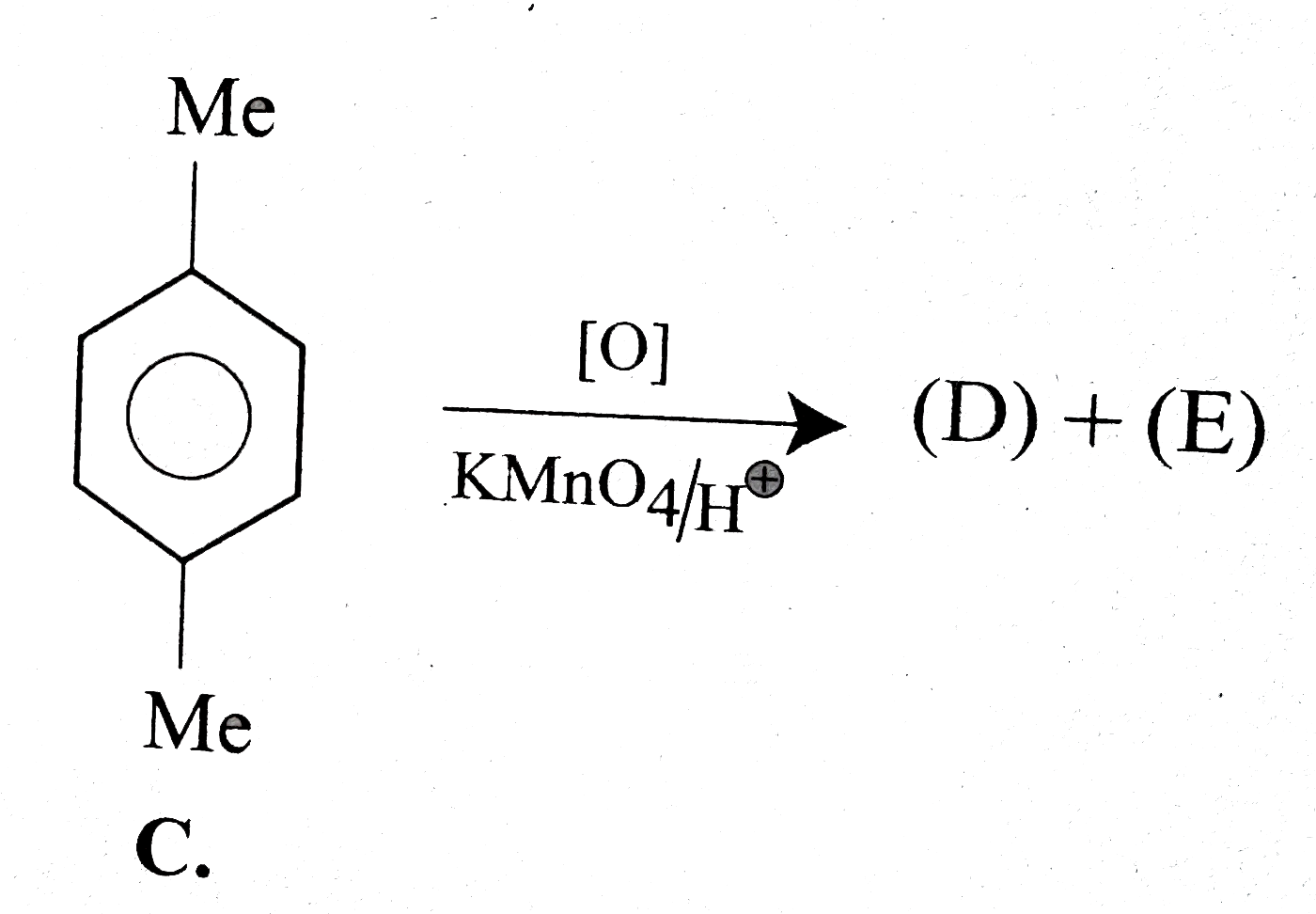 .
.
The compound D is an ortho-isomer and E is a p−isomer.ThecompoundsDandE, respectively, are :
View Solution