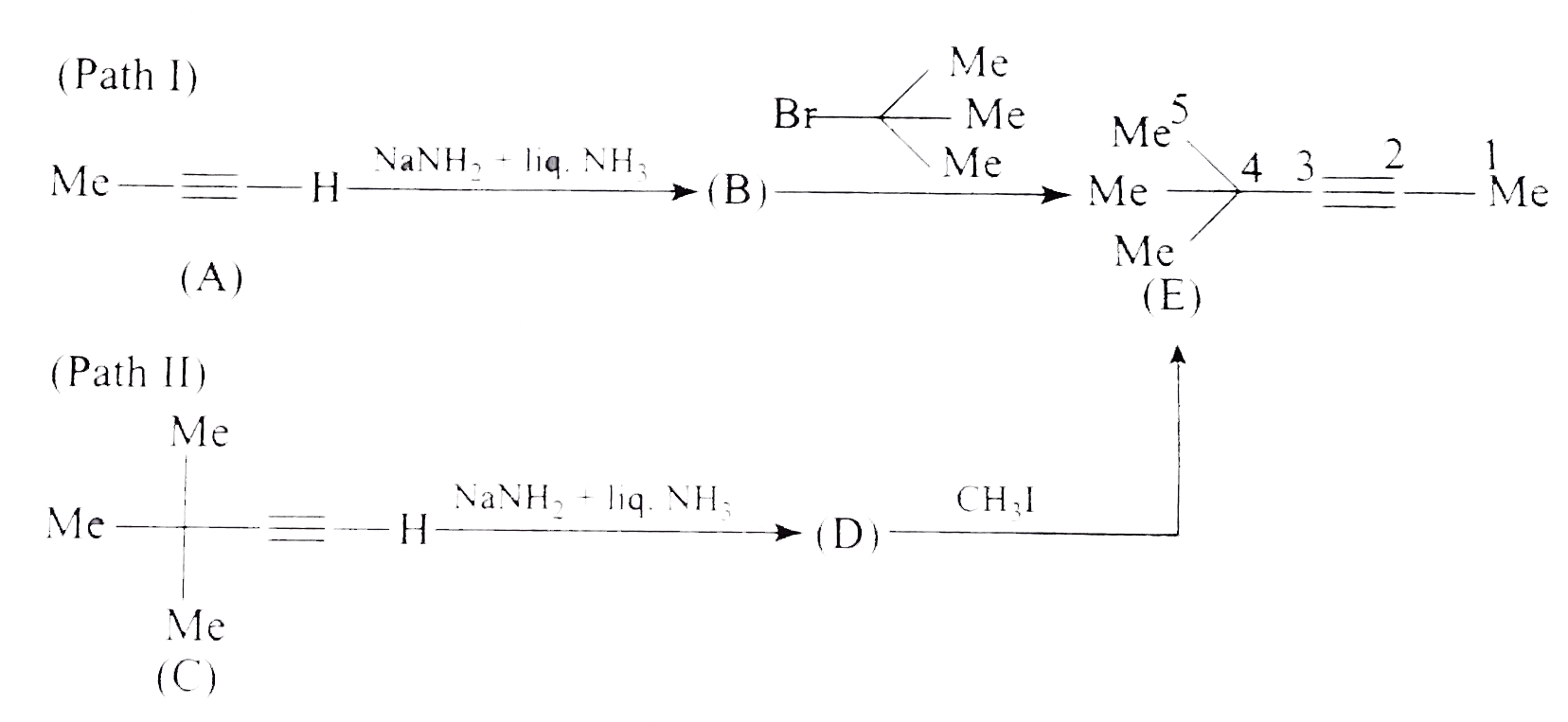
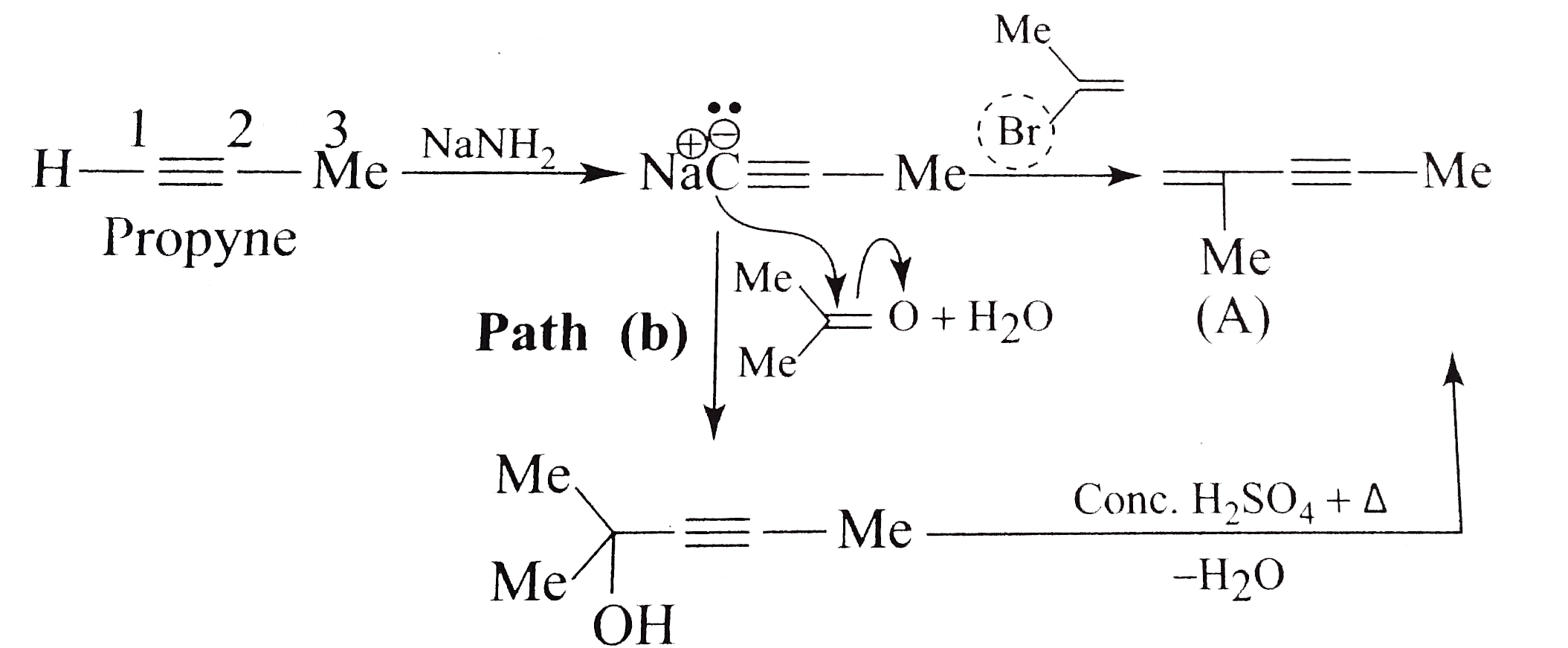
There are two paths (a) and (b) for the preparation of a compound (A) 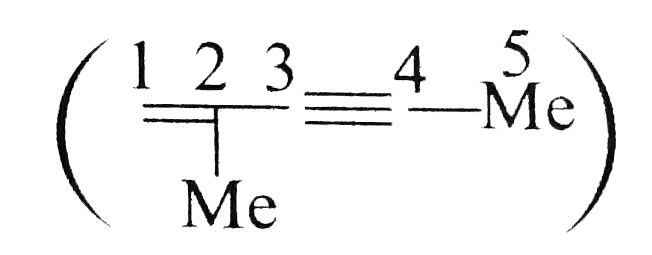 (2-methylpent-1-en-3-yne), which path is correct and why? Also name the path (a) and (b).
(2-methylpent-1-en-3-yne), which path is correct and why? Also name the path (a) and (b).
Video Solution
Text Solution
Path (b) is correct because in path (a) compound 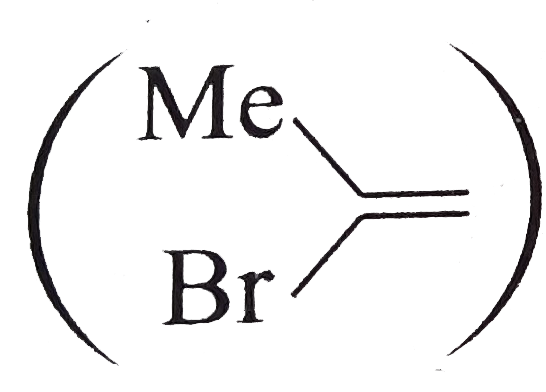 is vinyl halide which is not reactive, so reaction does not occur.
is vinyl halide which is not reactive, so reaction does not occur.
Path (a) is the alkylation of alkyne, whereas path (b) is propinylation (type of ethinylation), i.e., addition of propyne to unsaturated link like (>C=O) group.
|
Similar Questions
Knowledge Check
Correct representation of 3- methylpent-3-en-2-ol is
A<imga≤ocr_imageload∈g=lazysrc=https://d10lpgp6xz60nq.cloudfront.net/physics_images/NTA_NEET_SET_62_E02_008_O01.pngwh=80%mx-autosty≤=background:white;>B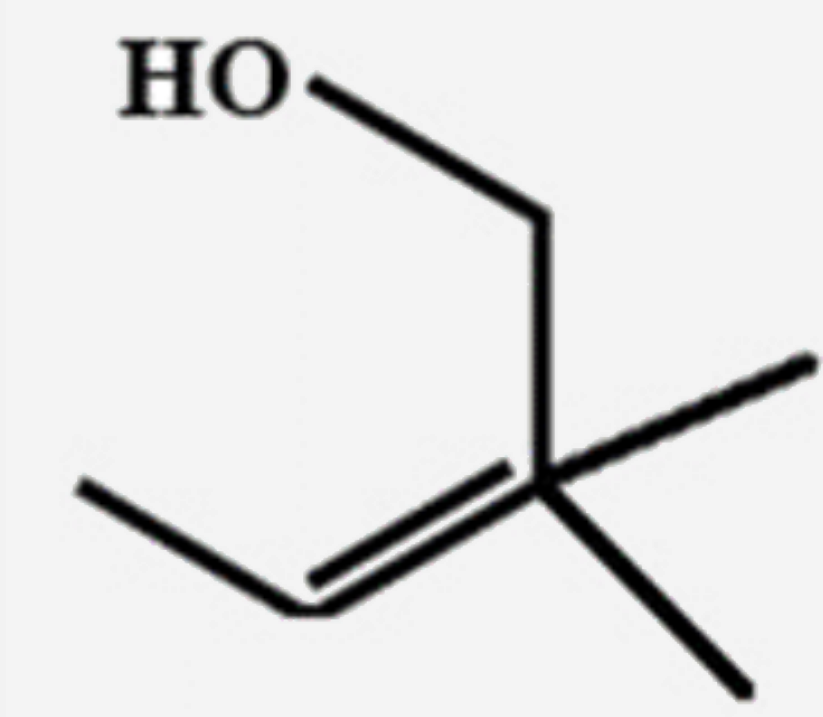 C
C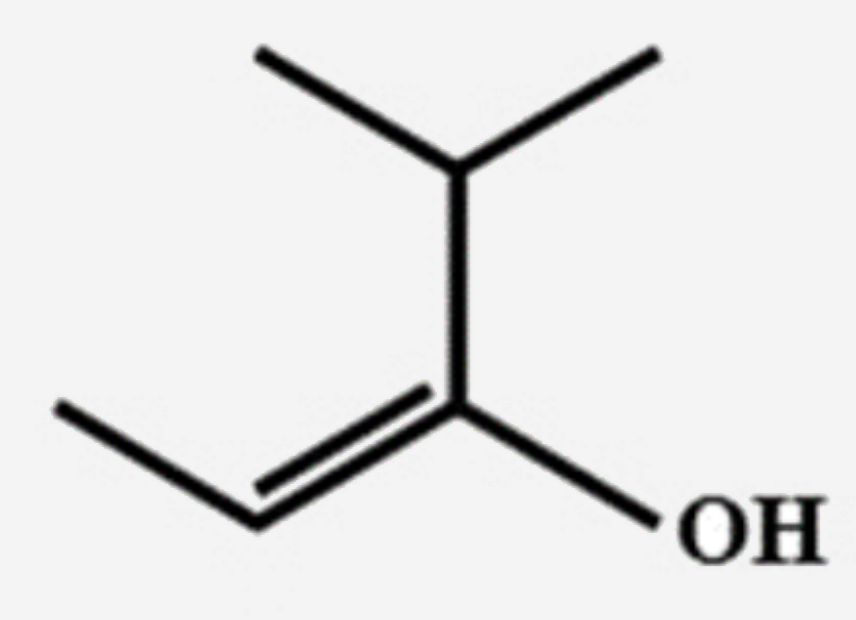 D
D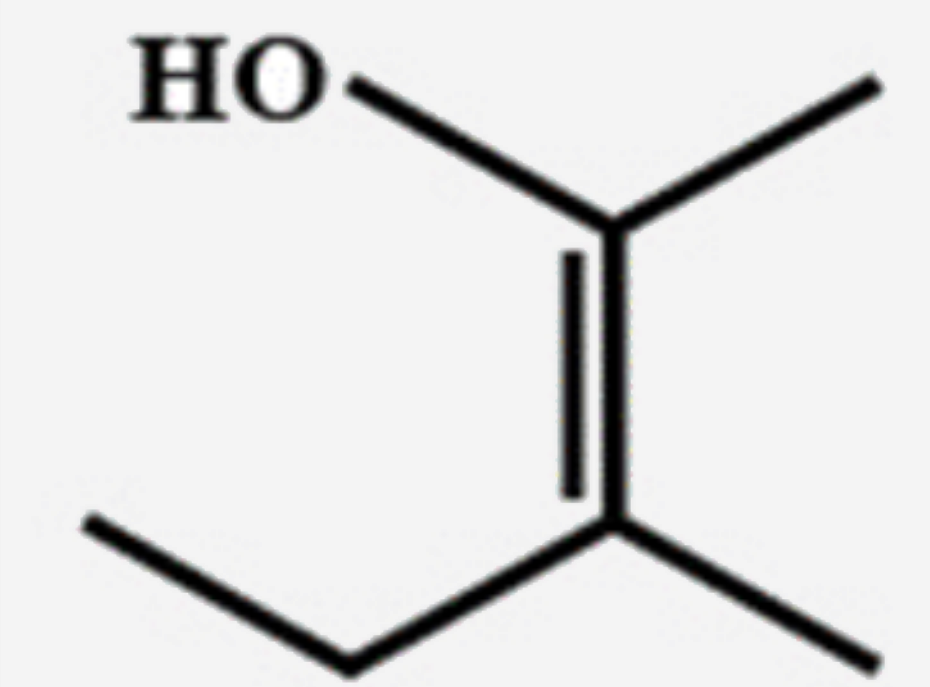
Which of the following paths is/are feasible for the preparation of ether (A) ?
A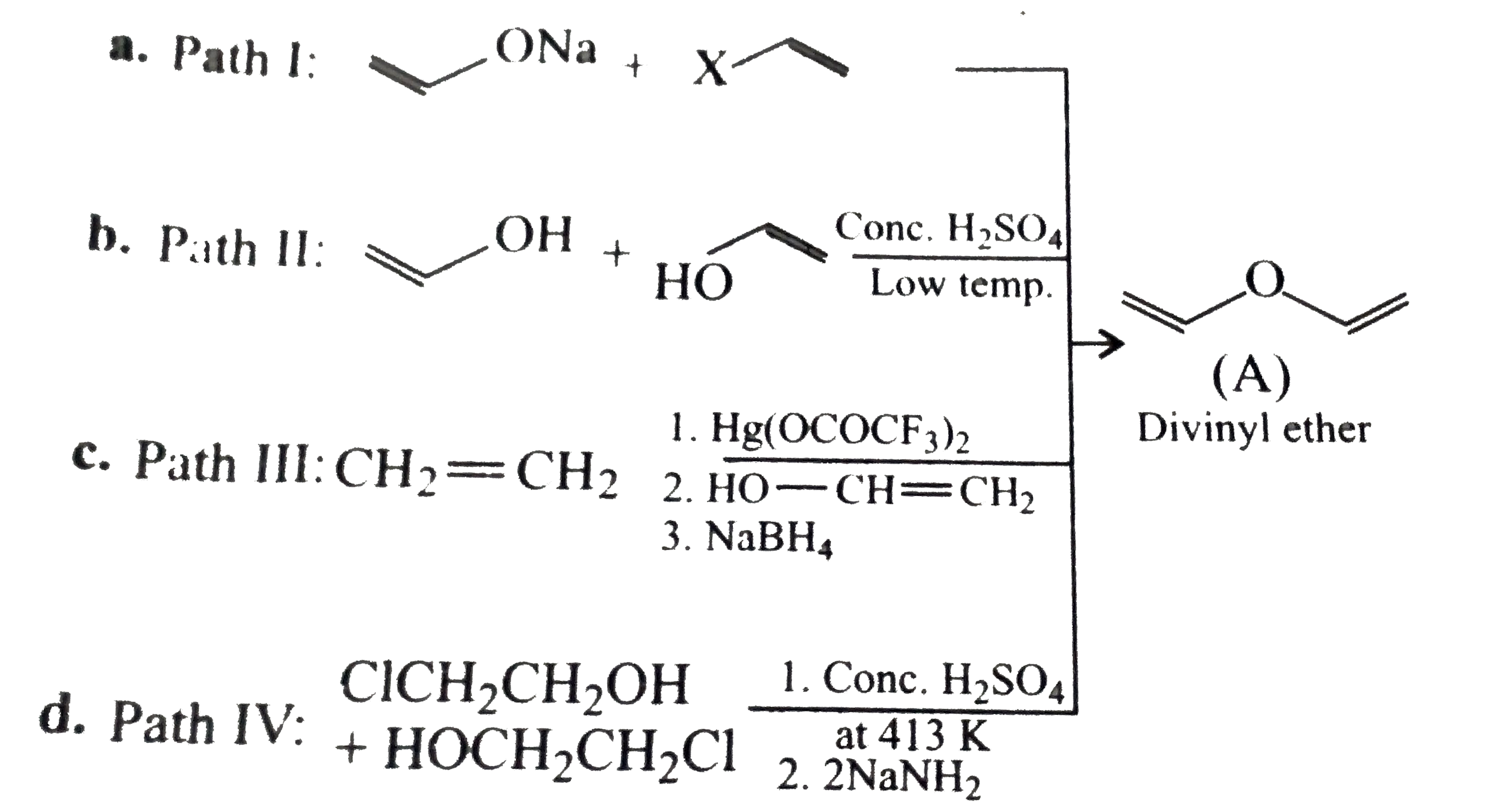 B
B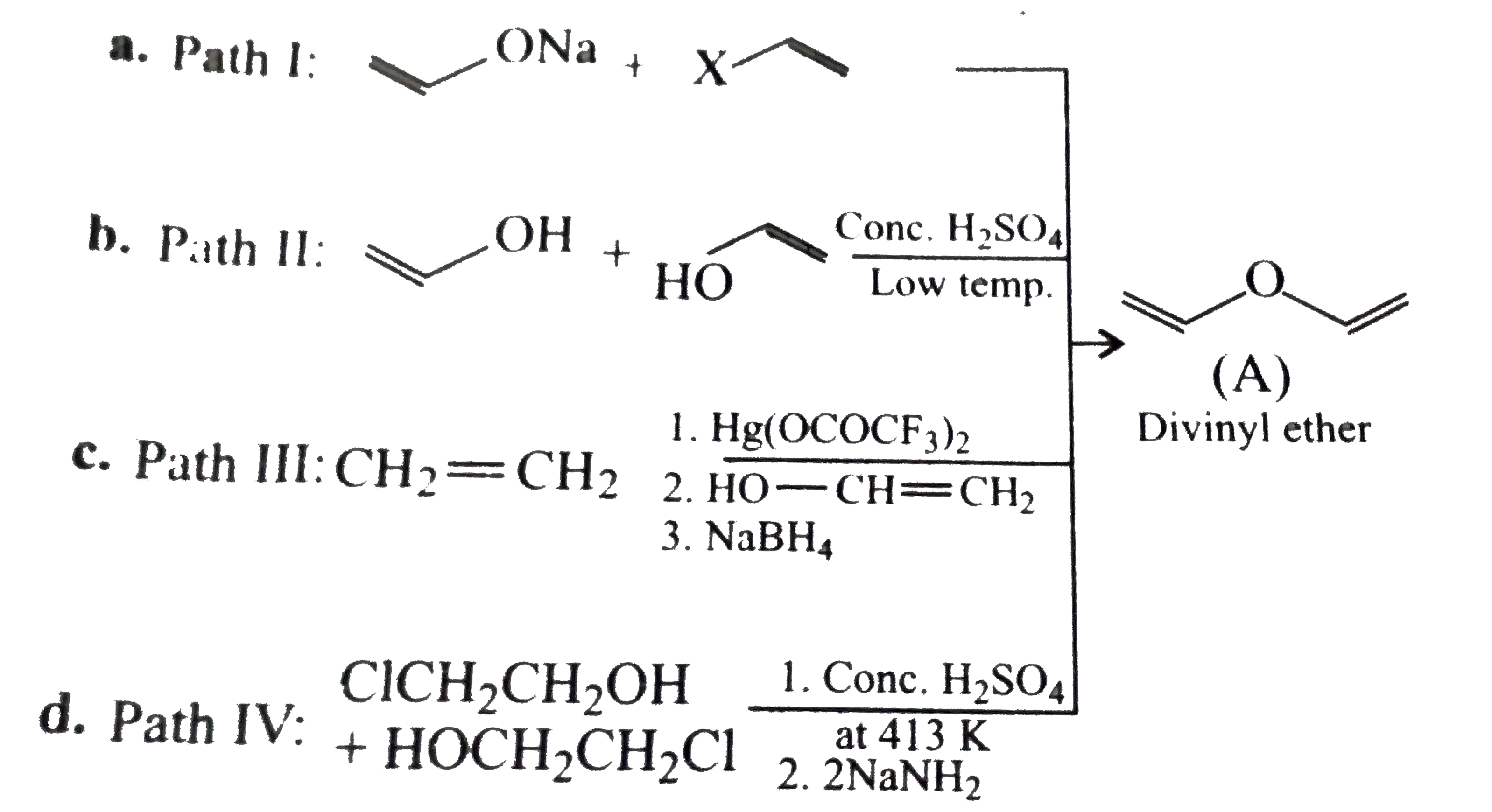 C
C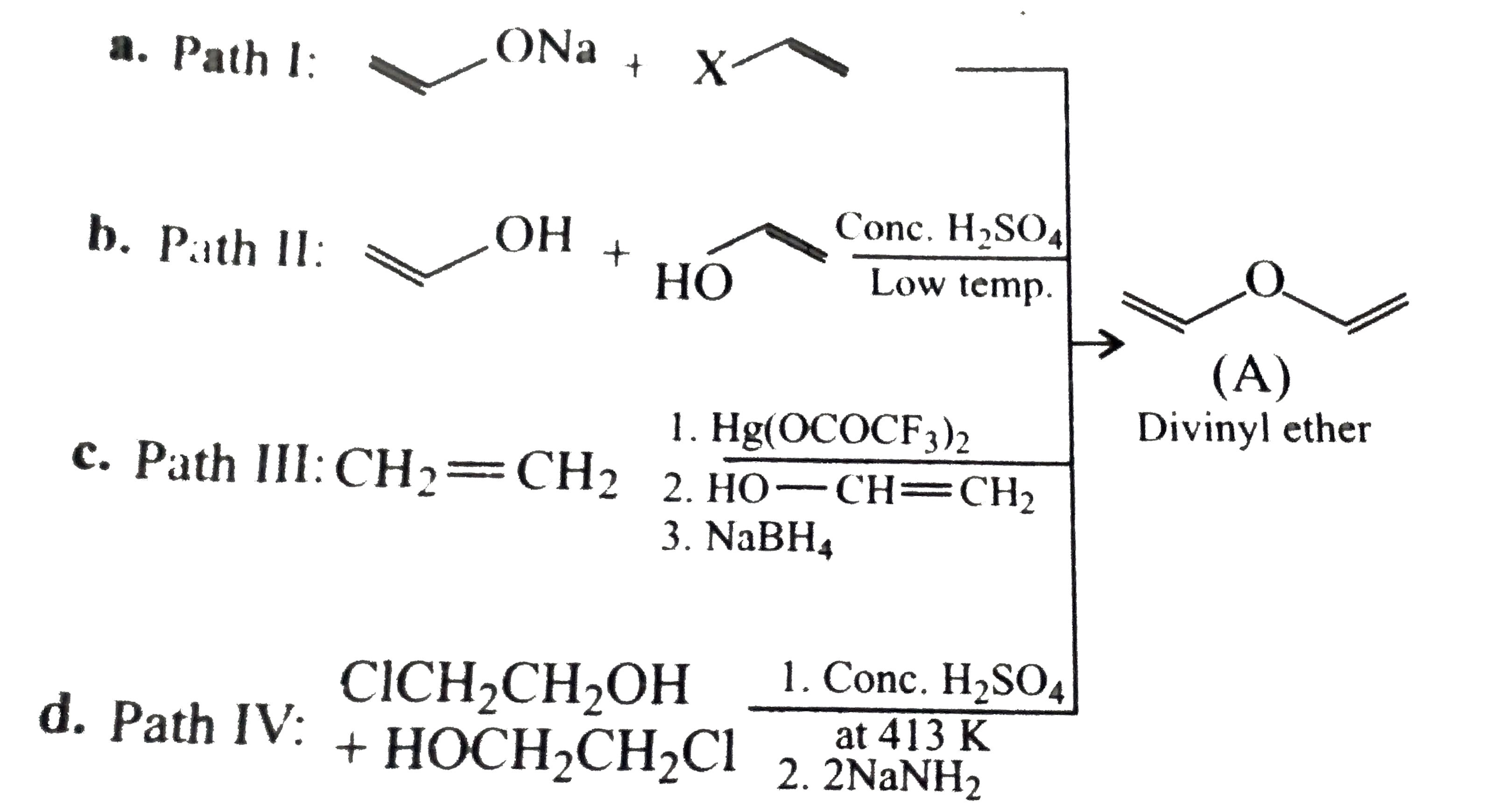 D
D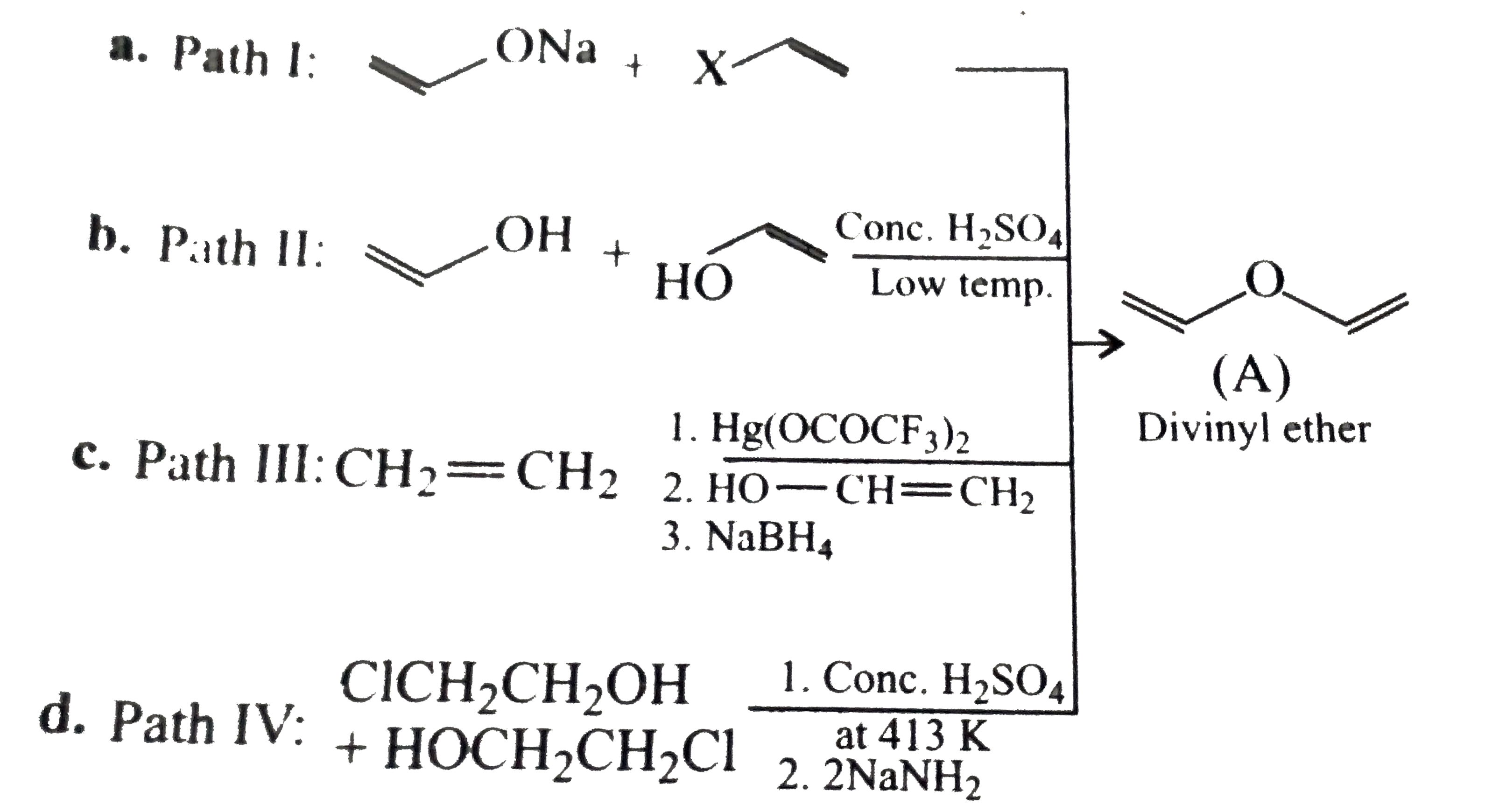
The correct representation of 4-hydroxy-2-methylpent-2-en-1-al is
ACH3−C∣OHH−CH=C∣CH3−CHOBCH3−C∣CH3H−CH=C∣OH−CHOCCH3−CH3∣C∣OH−CH=C∣CH3−CHODCH3−C∣OHH−CH2−C∣CH3H−CHO
There are two paths for the preparation of 2−methyl pentanoic acid (III) from pentan−2−ol(I). Which path is feasible and why ?
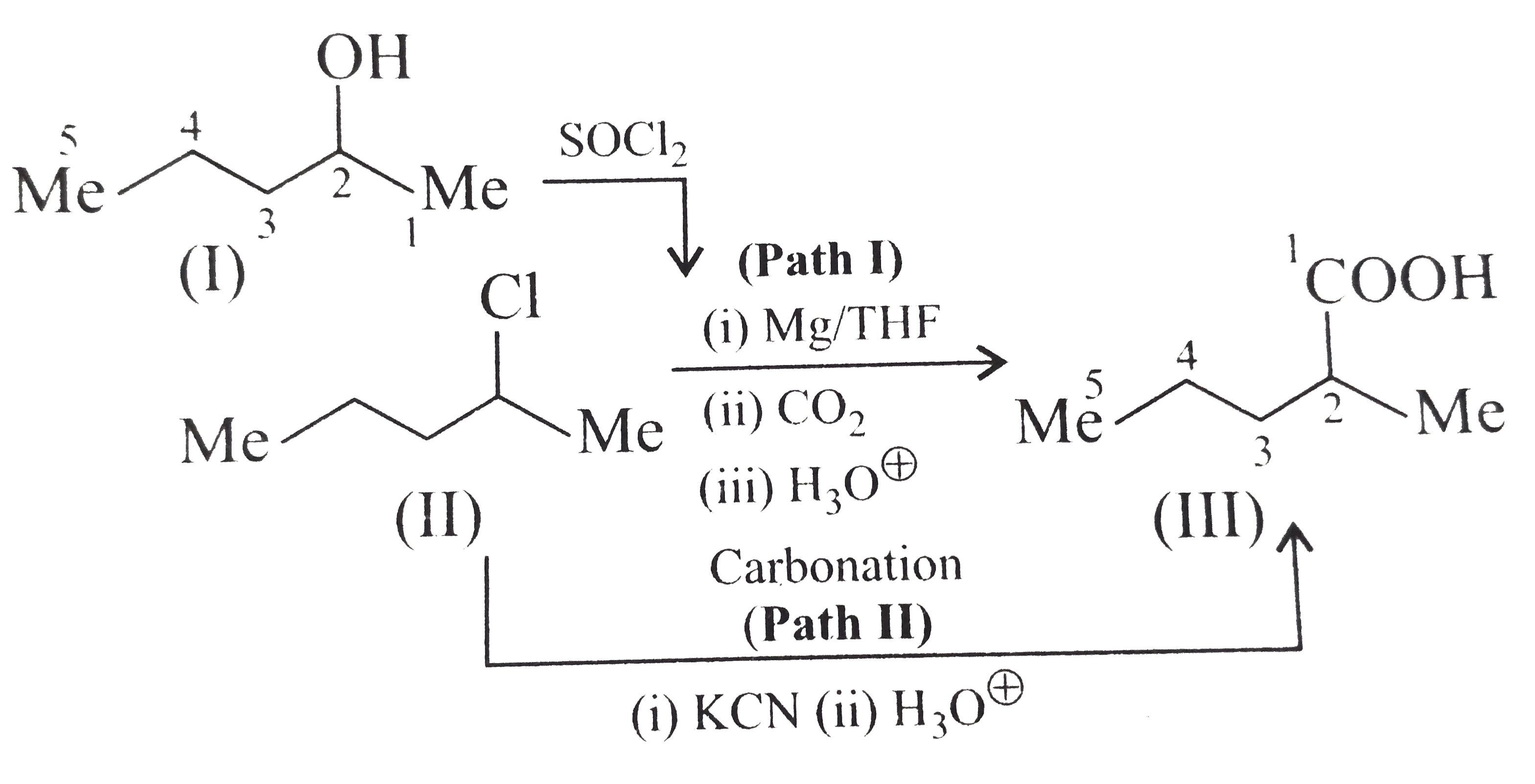 .
.
View SolutionThere are two paths for the preparation of phyebnyl-2, 4-dinitro phenyl ether (C). Which path is feasible and why ?
Path I :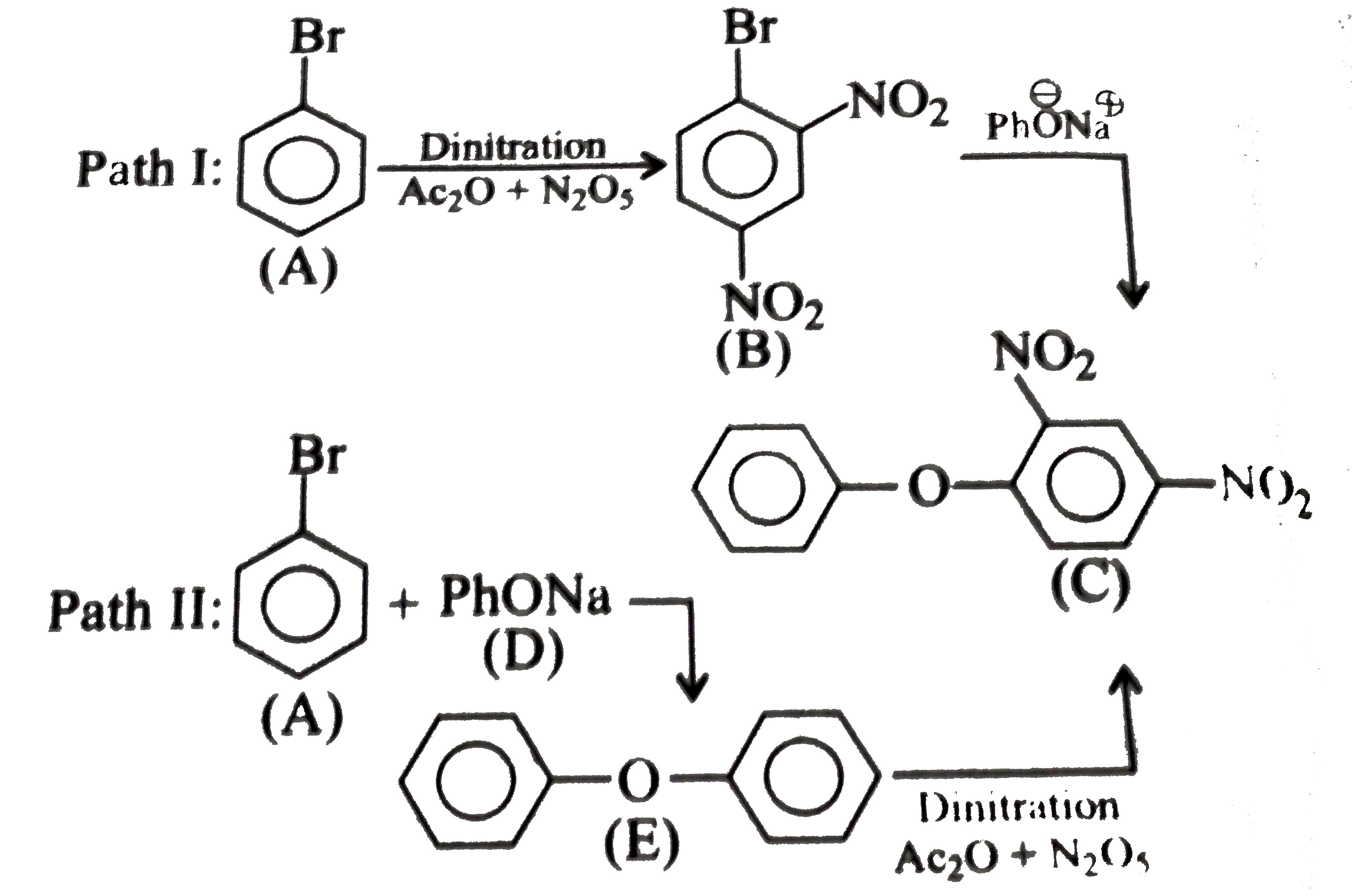
View SolutionThere are two paths to prepare compound (C).

Which path is feasible and why?

View SolutionWhich of the following path is are feasible for the preparation of the ether (A) ?
View SolutionWhich of the followign paths is/are feasible for the preparation of ether (E ) ?
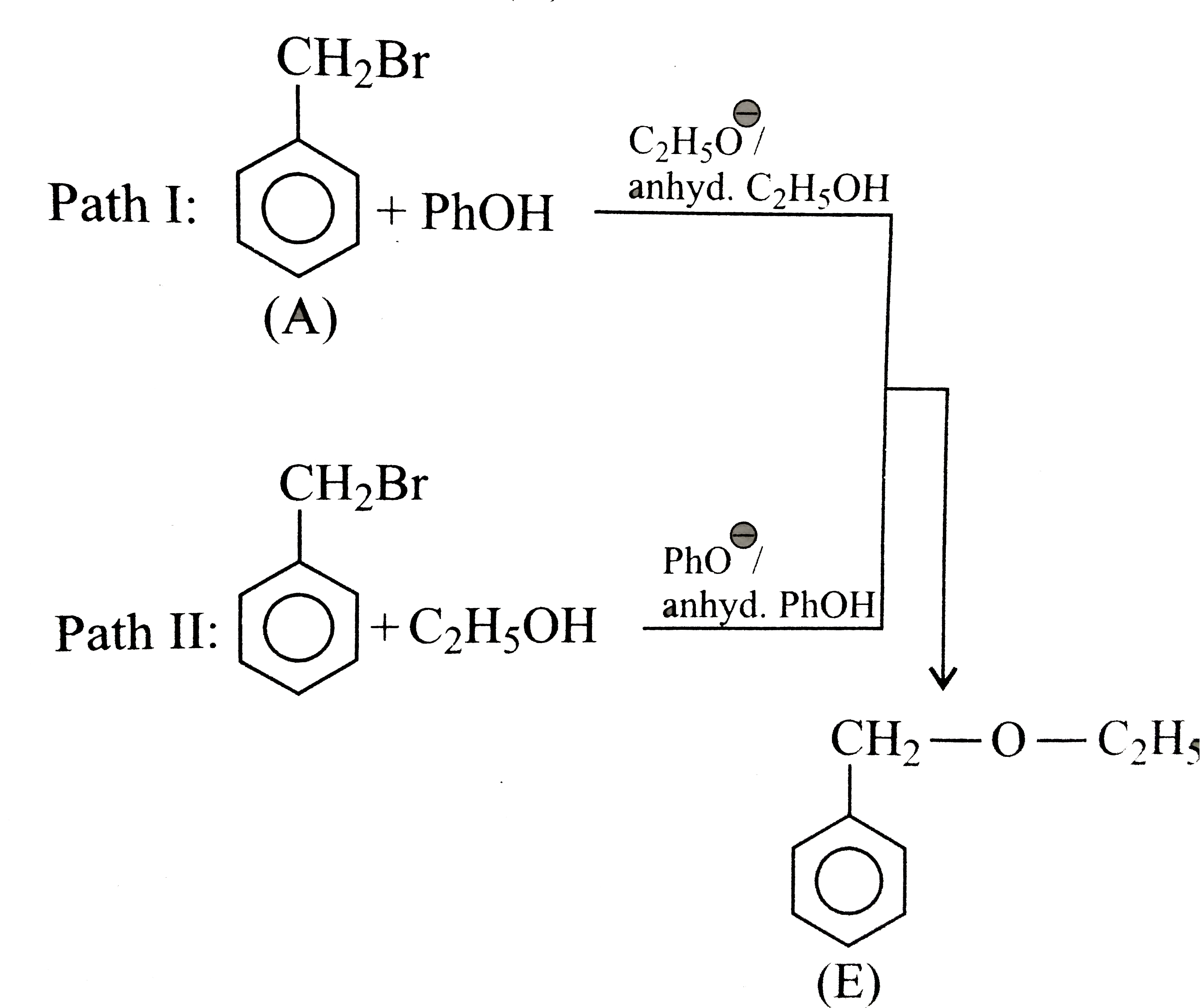
View Solution