What are the geometrices of:
i. Prop-1-yne
ii. But-2-yne
iii. Hept-2-en-5-yne
Video Solution
Text Solution
The shape of molecule depends on the nature of hybridisation of 'key' atoms.
i. Me−C≡C−H, sp hybridisation, linear molecule
ii. Me−C≡C−Me, sp hybridisation, linear molecule
iii. 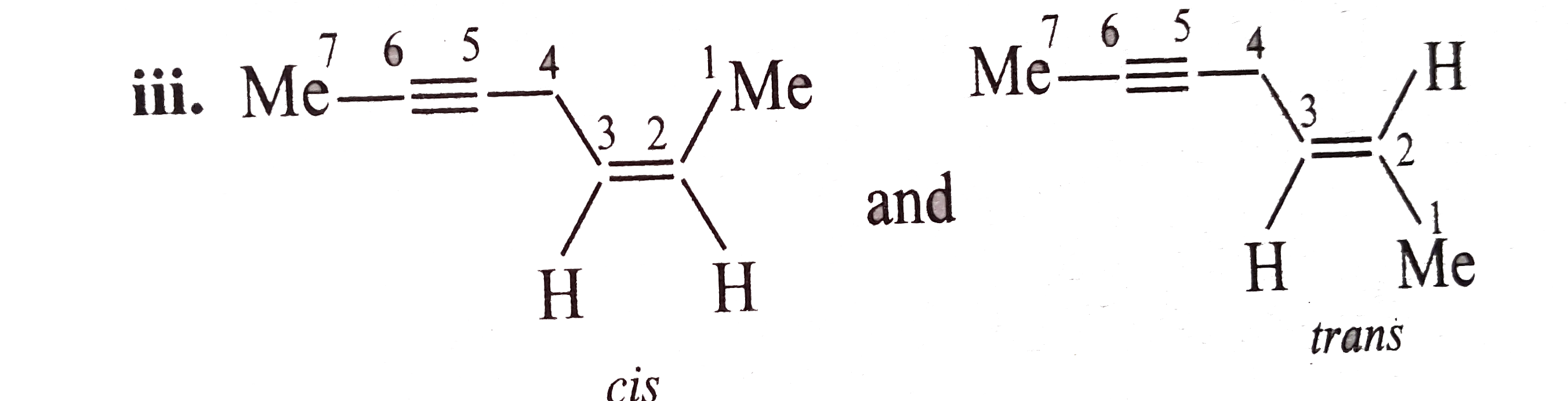
Double bond is given preference, sp2 hybridisation, and is trigonal or planar in shape.
|
Similar Questions
Knowledge Check
Hexa-2-ene-4-yne is
ACH3−CH2−C≡C−CH=CH2BCH3−C≡C−CH=CH−CH3CCH3CH2−CH=CH−C≡CHDCH3−C≡C−CH2−CH=CH2Hexa-2-ene-4-yne is
ACH3−CH2−C≡C−CH=CH2BCH3−C≡C−CH=CH−CH3CCH3CH2−CH=CH−C≡CHDCH3−C≡C−CH2−CH=CH2But- 2-yne contains :
Asp hybridised carbon atoms onlyBsp3 hybridised carbon atoms onlyCboth sp and sp2 hybridised carbon atomsDboth sp and sp3 hybridised carbon atoms
Which of the following alkynes react with sodium in liquid ammonia ?
(i) Propyne (ii) But-2-yne (iii) Pent-3-yne (iv) Hex-1-yne
View SolutionWrite the structures of the following compounds :
(i) But-2-enoic acid
(ii) But-1-en-3-yne
(iii) 3, 4, 4-Trimethylhex-1-yne
View SolutionBut-1-yne and But-2-yne can be distinguished by :
View SolutionNumber of sp hybridised carbon atoms in But-2-yne is
View SolutionNumber of acidic hydrogen atoms in but-1-yne is
View Solution

