Explain or complete the following reactions.
(i) 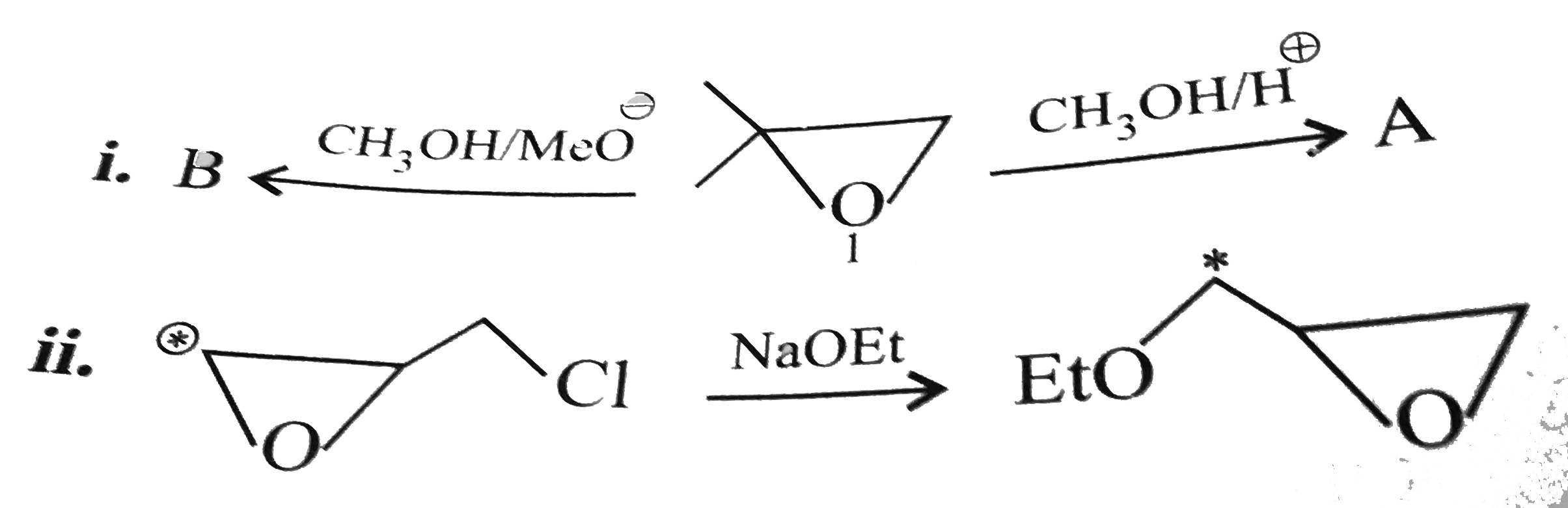
Video Solution
Text Solution
(i) In a base-catalysed reaction, ring opening takes place via SN2 mechanism. In an acid-catalysed reaction, ring opening takes place vua SN1 mechanism to give more stable 3∘C⊕.
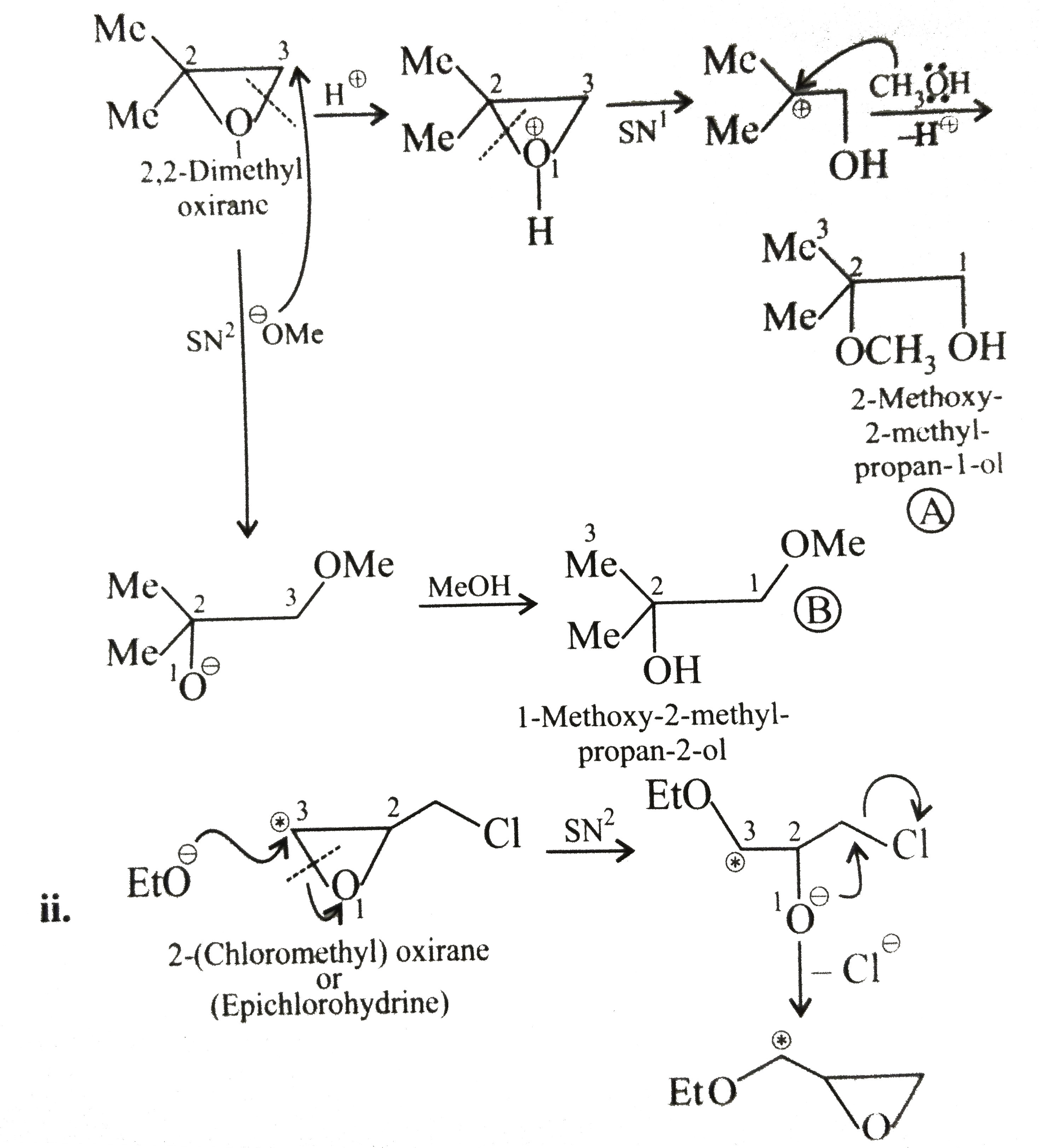 .
.
This is not a direct Williamson's reaction. Instead, the reaction is initiated by the SN2 displacement by EtOӨ on the less substituted C∗(C14) of the epoxide giving an intermediate alkoxide ion. The OӨ then acts as a neighbouring group participant to displace ClӨ by a second SN2 reaction forming the product.
|
Topper's Solved these Questions
- GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTSBook:CENGAGE CHEMISTRYChapter:GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTSExercise:Solved Examples
Explore 15 Videos
- GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTSBook:CENGAGE CHEMISTRYChapter:GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTSExercise:Exercises (Subjective )
Explore 17 Videos
- GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTSBook:CENGAGE CHEMISTRYChapter:GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTSExercise:Archives (Subjective)
Explore 14 Videos
- NCERT BASED EXERCISEBook:CENGAGE CHEMISTRYChapter:NCERT BASED EXERCISEExercise:Nuclear Chemistry (NCERT Exercise)
Explore 29 Videos
Similar Questions
Complete the following reactions :
(a)
View SolutionComplete the following reactions:
(I) a.
View SolutionComplete the following reactions: a.
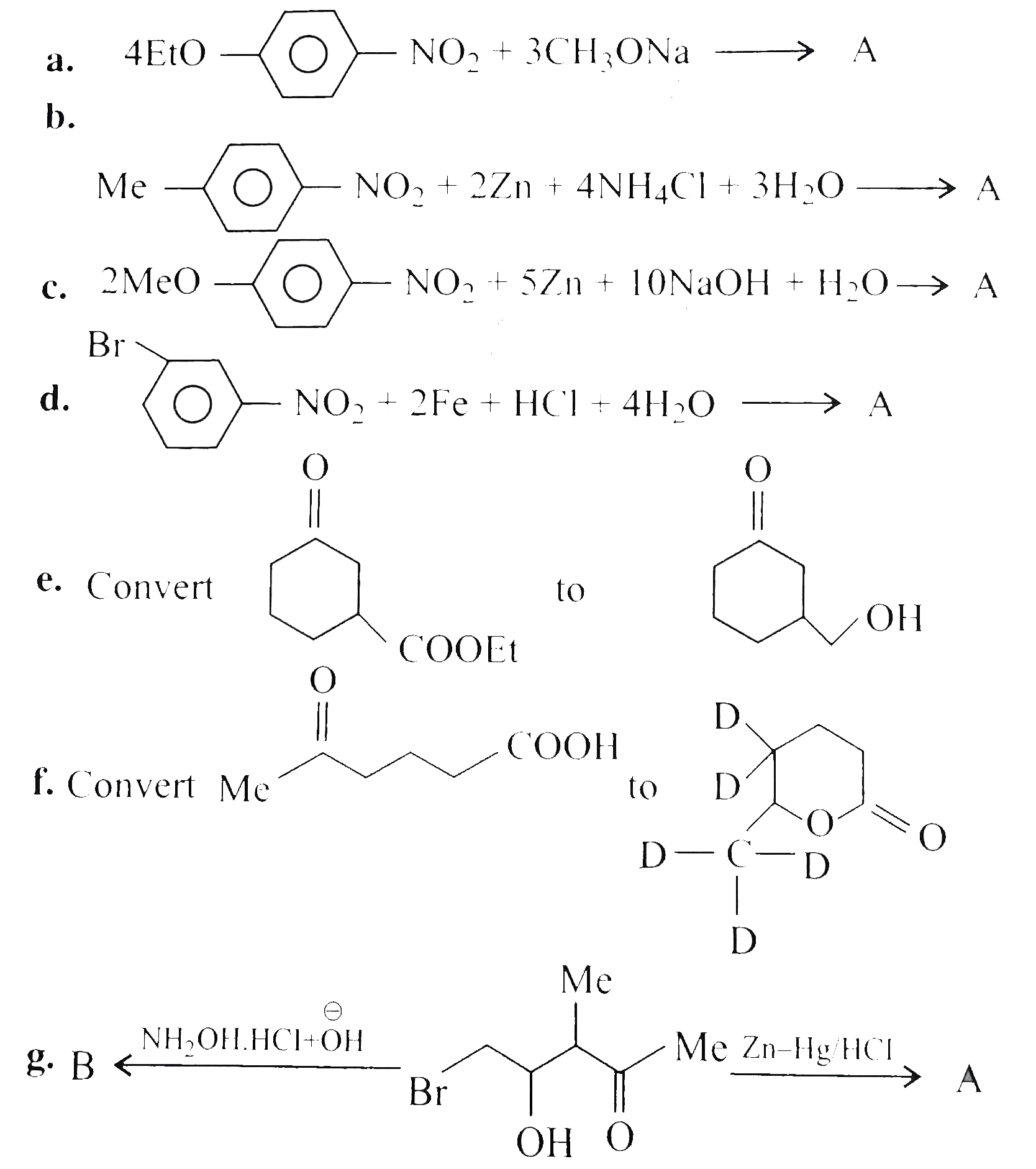
View SolutionComplete the following reactions:
a.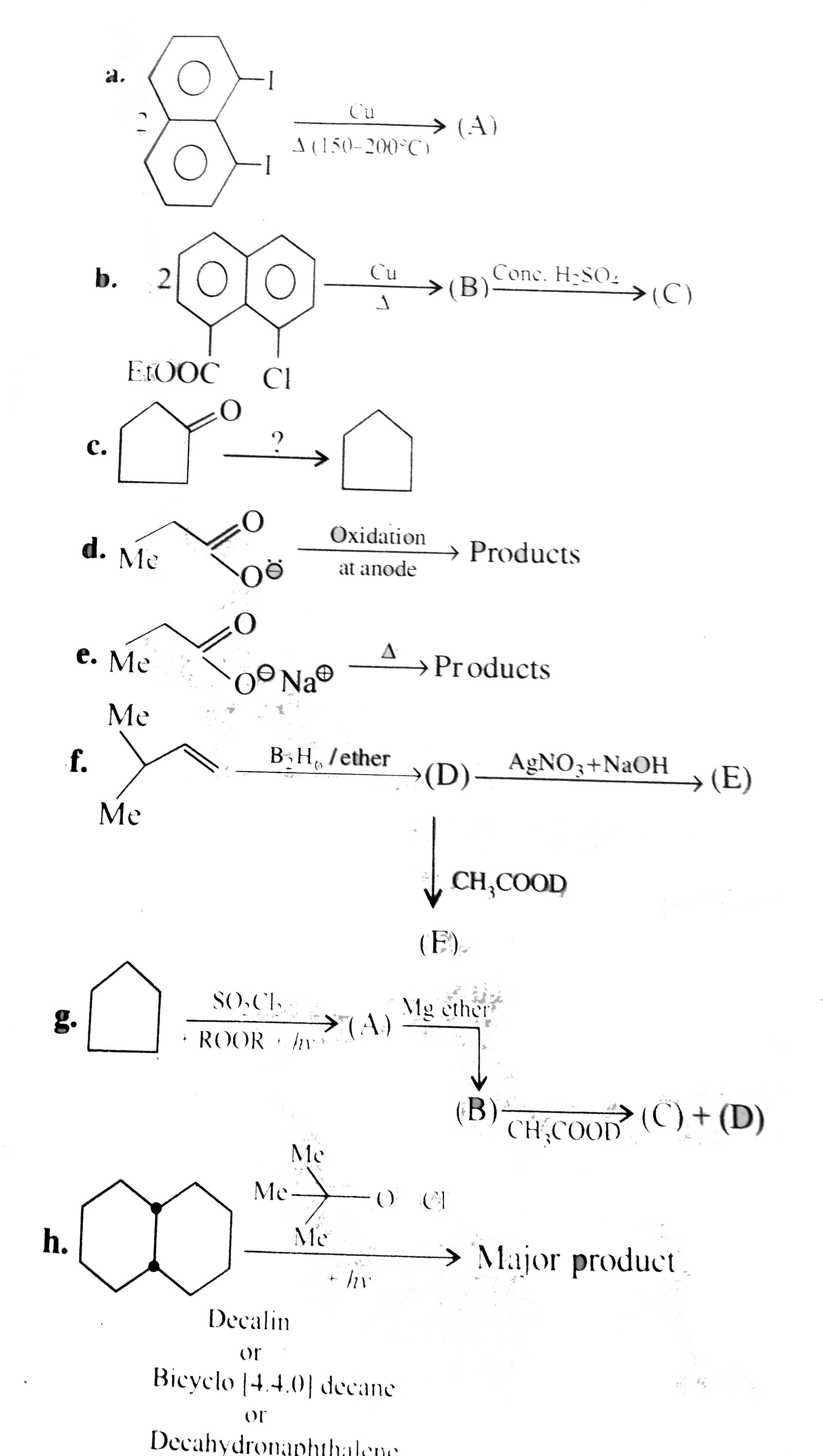
View SolutionComplete the following reactions :
a.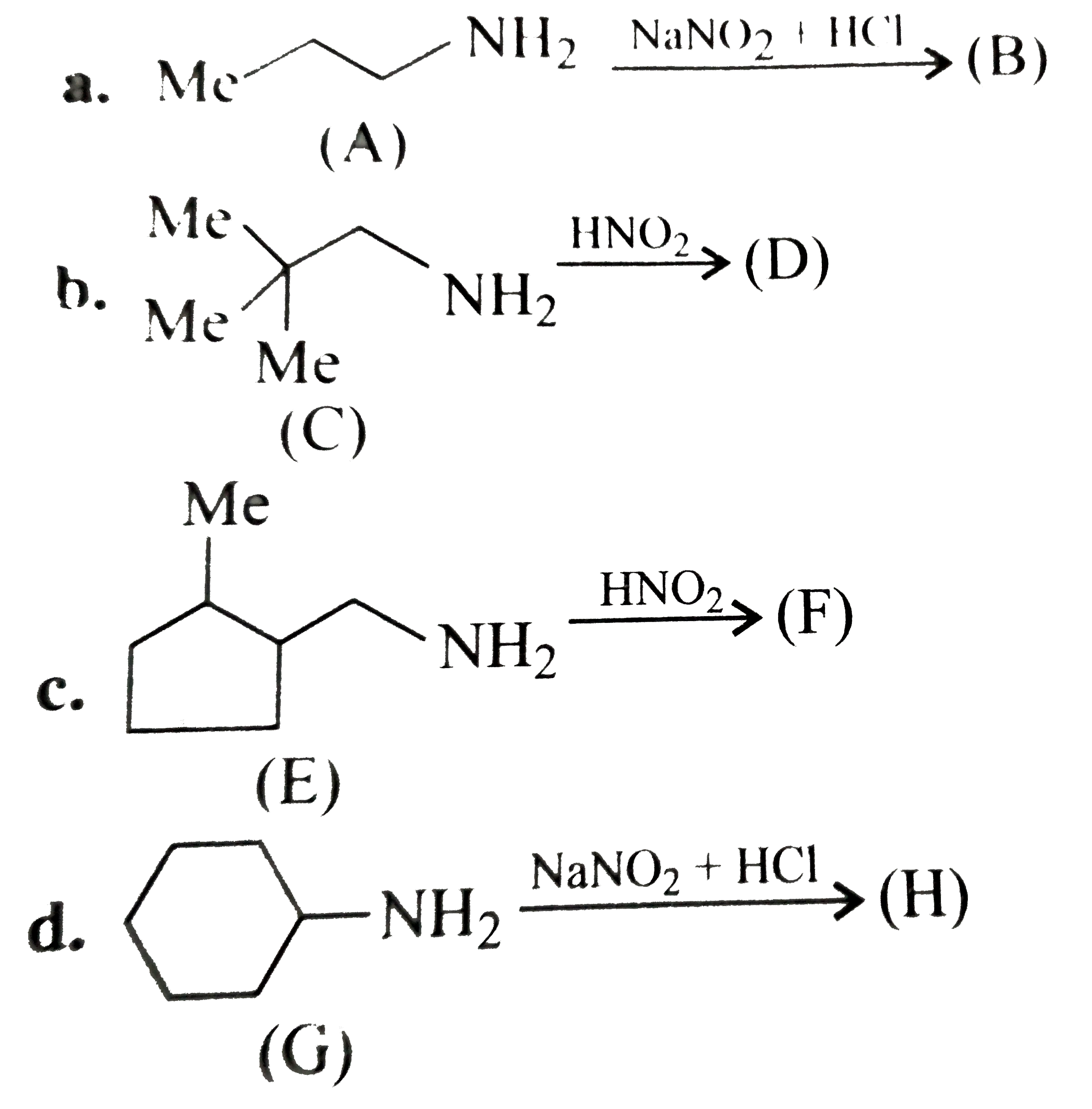
View SolutionComplete the following reactions :
i.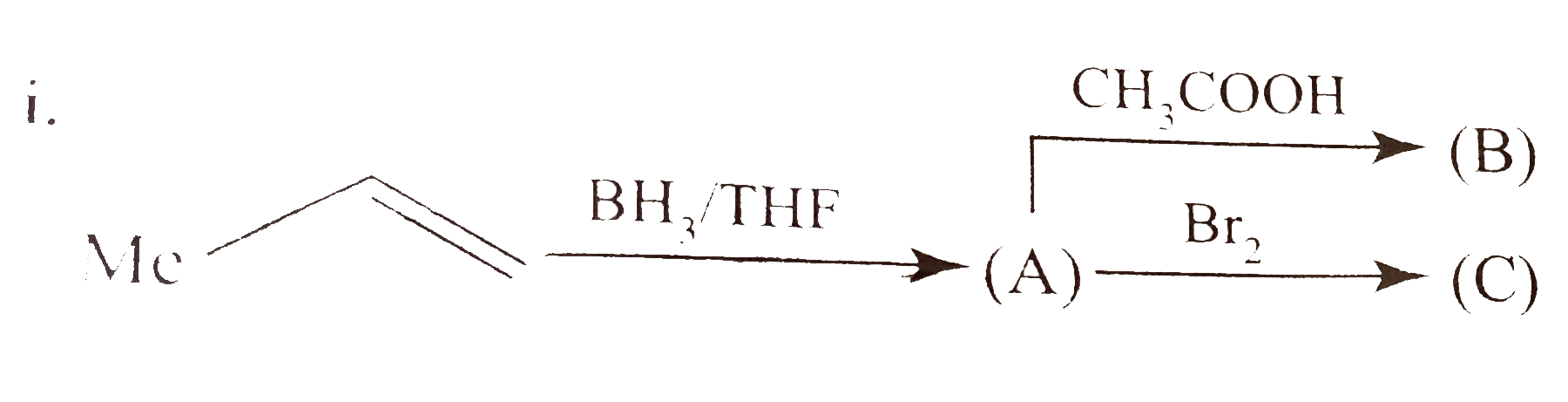
ii.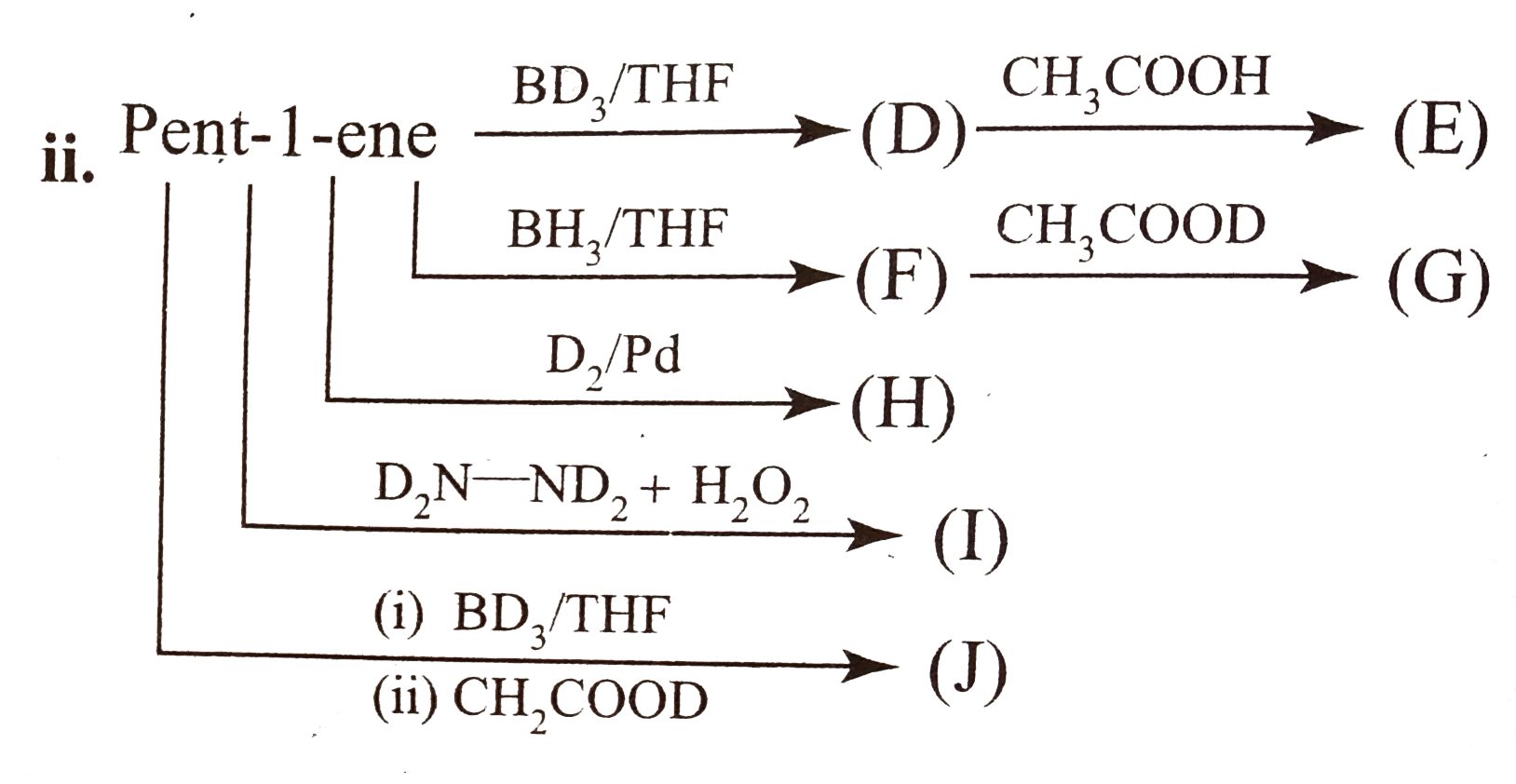
View SolutionComplete the following reactions :
i.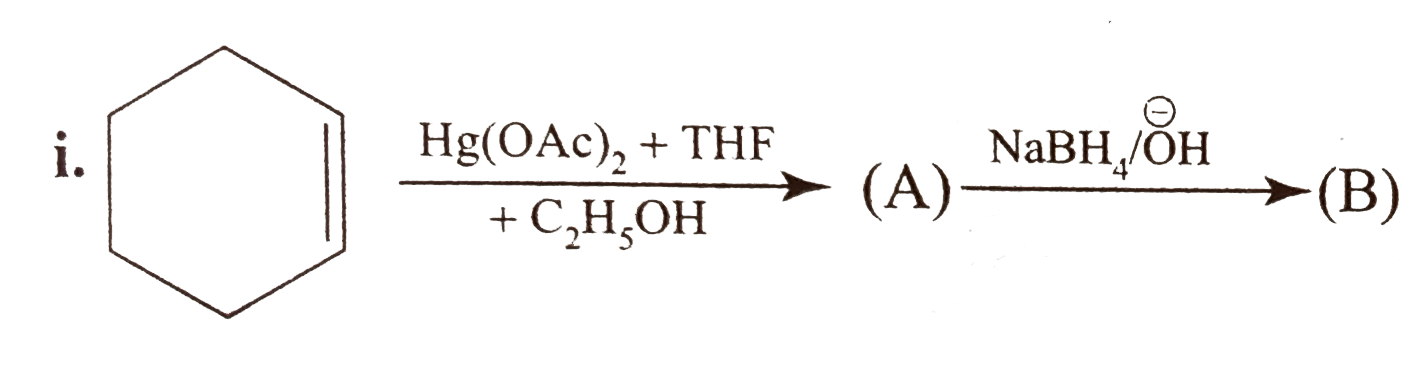
ii.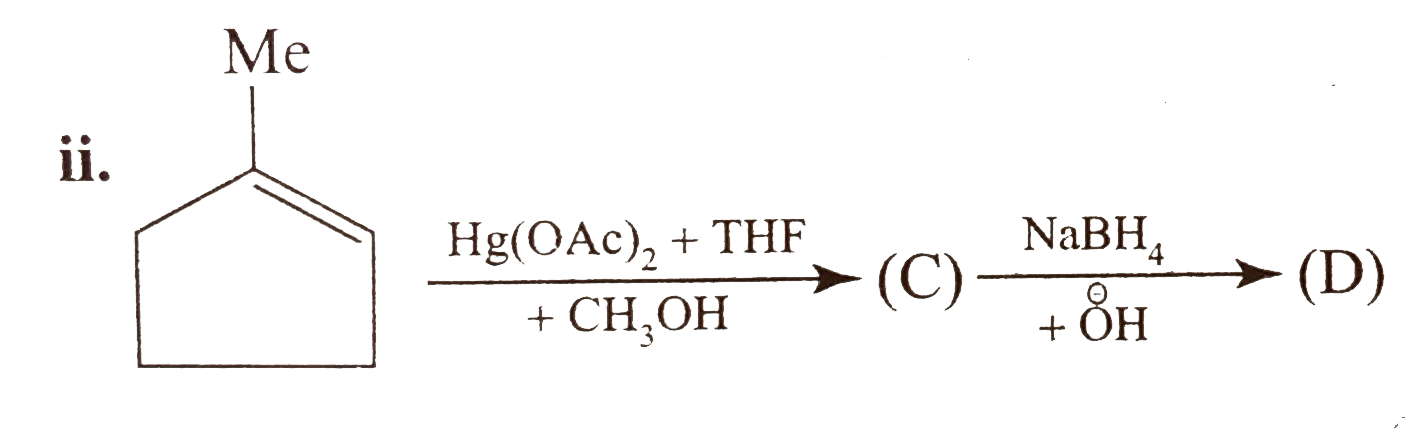
View SolutionComplete the following reactions :
(a)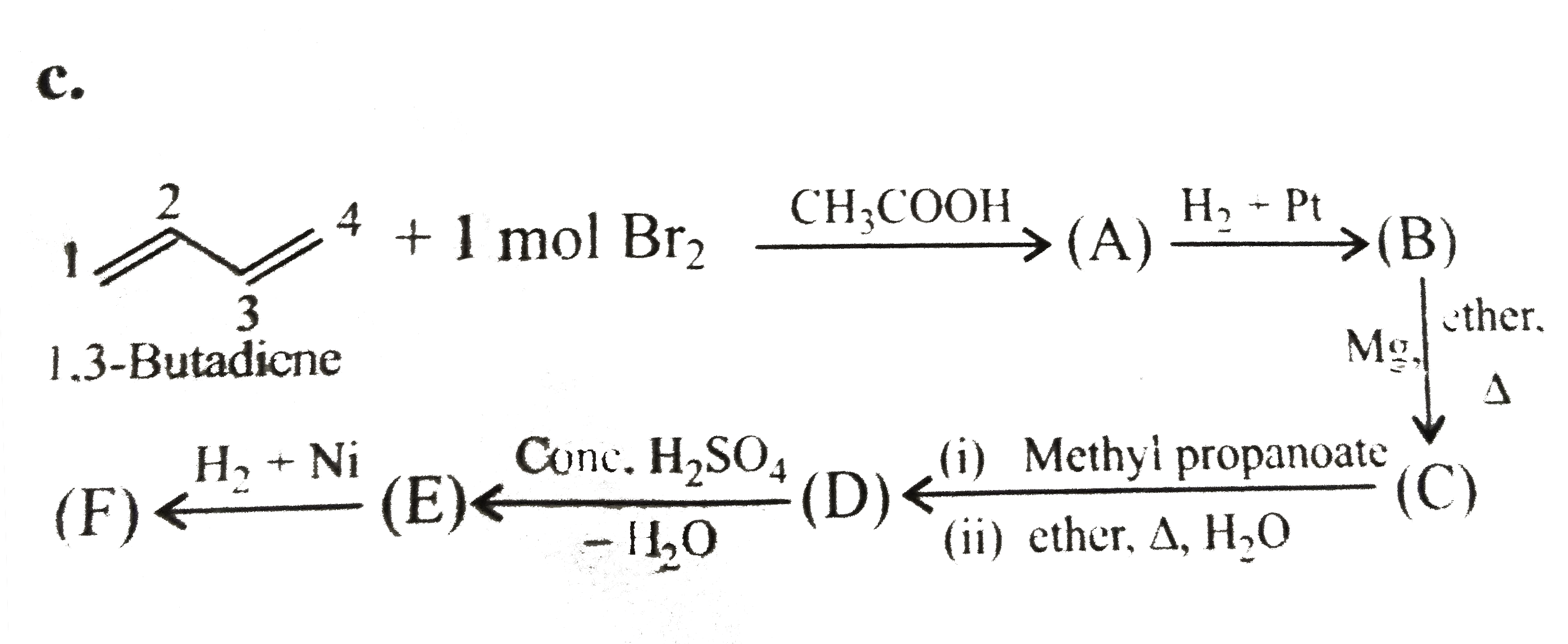
(b)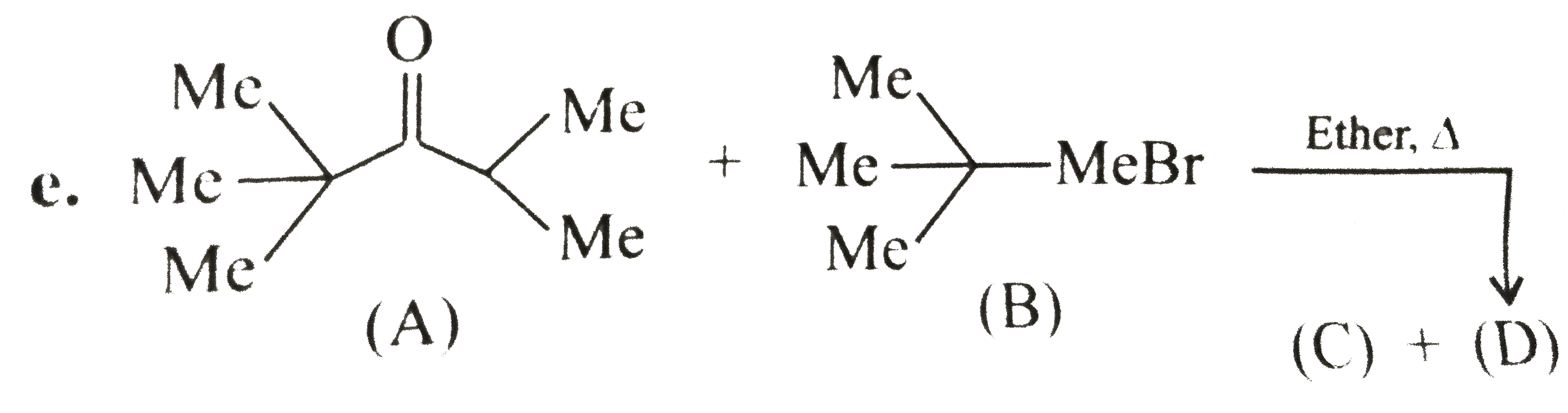
View SolutionComplete the following reactions:
(a)_S01_417_Q01.png) (b)
(b) _S01_417_Q01.png)
View SolutionComplete the following reaction:
i.
ii.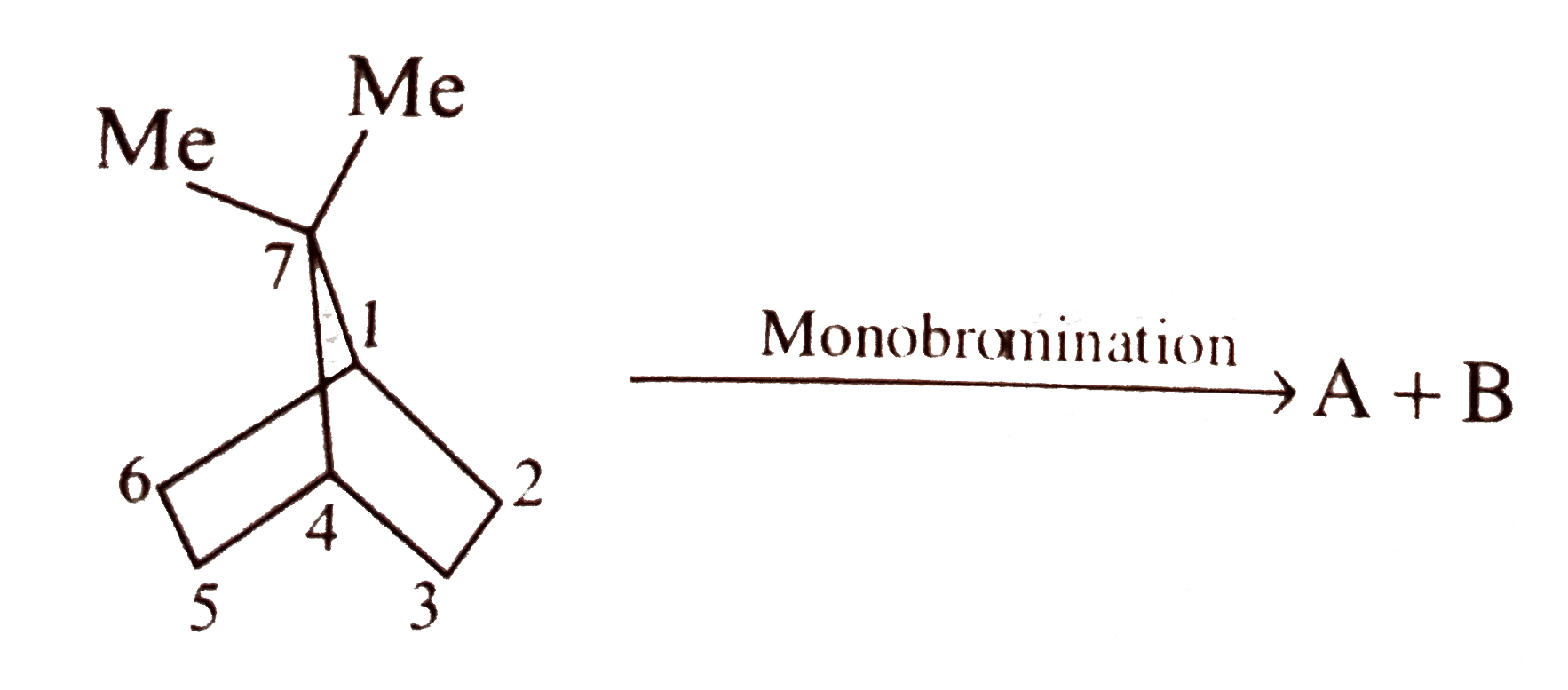
View Solution
