EtNH2+MeMgIHeated at high temp.−−−−−−−−−−−−−−−→in the presence of pyri di ne Gas(A) The volume of gas (A) obtained at S.T.P when 0.45gm of EtNH2 reacts with MeMgI is.
224ml
22.4ml
448ml
44.8 ml
The correct Answer is:C
( c) Only one hydrogen atom in 1∘RNH2 reacts at room temperature. In the presence of pyridine, both H atoms in 1∘RNH2 react.
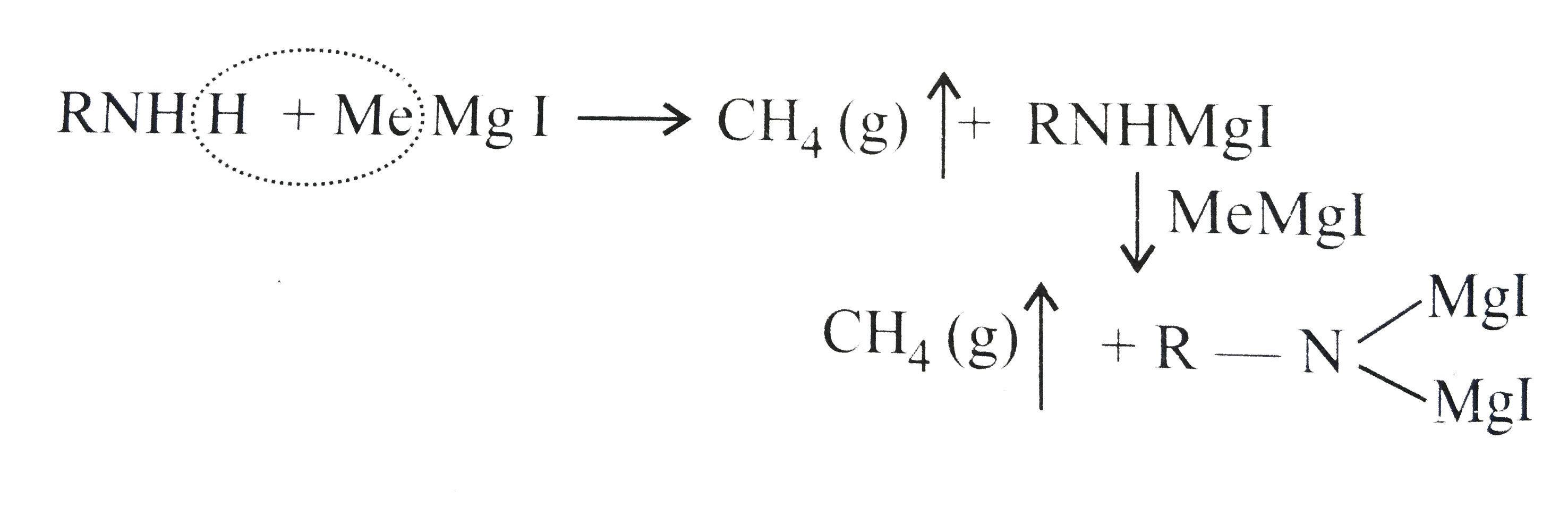 .
.
One mole of EtNH2 reacts with 2mol of MeMgI to give 2mol of CH4(g). Molecular mass of EtNH2 is 45gm.
Therefore, 45gm of EtNH2 gives 2×22.4litres of CH4 at S.T.P
0.45gmofEtNH2gives2×22.4×0.4545=0.448litres
=448ml.
Topper's Solved these Questions
- GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTSBook:CENGAGE CHEMISTRYChapter:GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTSExercise:Exercises (Single Correct)
Explore 47 Videos
- GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTSBook:CENGAGE CHEMISTRYChapter:GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTSExercise:Exercises Archives (Single Correct)
Explore 4 Videos
- GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTSBook:CENGAGE CHEMISTRYChapter:GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTSExercise:Exercises (Linked Comprehension)
Explore 33 Videos
- GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTSBook:CENGAGE CHEMISTRYChapter:GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTSExercise:Archives (Subjective)
Explore 14 Videos
- NCERT BASED EXERCISEBook:CENGAGE CHEMISTRYChapter:NCERT BASED EXERCISEExercise:Nuclear Chemistry (NCERT Exercise)
Explore 29 Videos
Similar Questions
1-Ethyl-2-methyl oxirane when treated with C2H5MgBr, followed by hydrolysis gives :
View SolutionMe3C−MgCl on reaction with D2O produces :
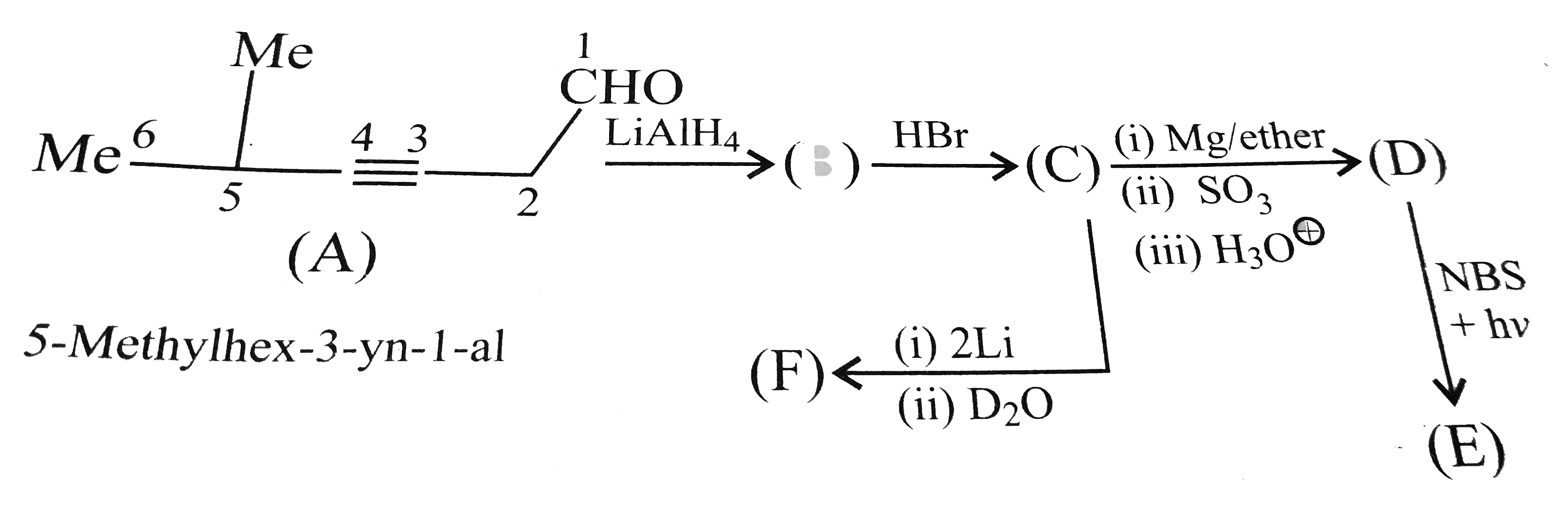
View SolutionIn the following reaction sequence, the products (B) to (E) are formed.
 .
.
The structure of product (B) is :
View SolutionWrite the chemical equations involved in the extraction of lead from galena by self-reduction process.
View SolutionClearly state, what do you understand by the terms : mass number, nucleaons and nuclides?
View SolutionIdentify the major products in the following reaction. C_6H_5 COOH + MeMgI rarr ? + ? .
View SolutionNa+Al_(2)O_(3) overset("High temp.") rarrXoverset(CO_(2)" in")underset("water")rarr Y, compound is
View Solution HI−−−−→low temp product are
HI−−−−→low temp product are
View Solution2.3 gm Na is added to 6 gm of CH3COOH. The volume of H2 gas liberated at S.T.P.
View SolutionThe vapour density of a gas is 11.2. The volume occupied by 11.2 gms of the gas at S.T.P is
View Solution'A'+NaOH→'B'(gas)+'C'Zn−−→'B'
'B'(gas)[O]−−→'D'(gas)Mg−−→Δ'E'(White)H2O−−→'B'(gas)
The gas D and B are respectively?
View Solution10 litres of O_(2) gas is reacted with 30 litres of CO at S.T.P. The volumes of each gas present at the end of the reaction are
View Solutionयदि डाइनाइट्रोजन गैस के 10 आयतन डाइ ऑक्सीजन गैस के 5 आयतनों से साथ अभिक्रिया करे , तो जलवाष्प के किंतने आयतन प्राप्त होंगे ?
View Solution367.5 gm KClO_(3) (M = 122.5) when heated, How many litre of oxygen gas is proudced at S.T.P.
View SolutionThe volume occupied by any gas at S.T.P. is ________
View Solution
