Ethyl ester MeMgBr−−−−−→excessP. The product P will be :
A
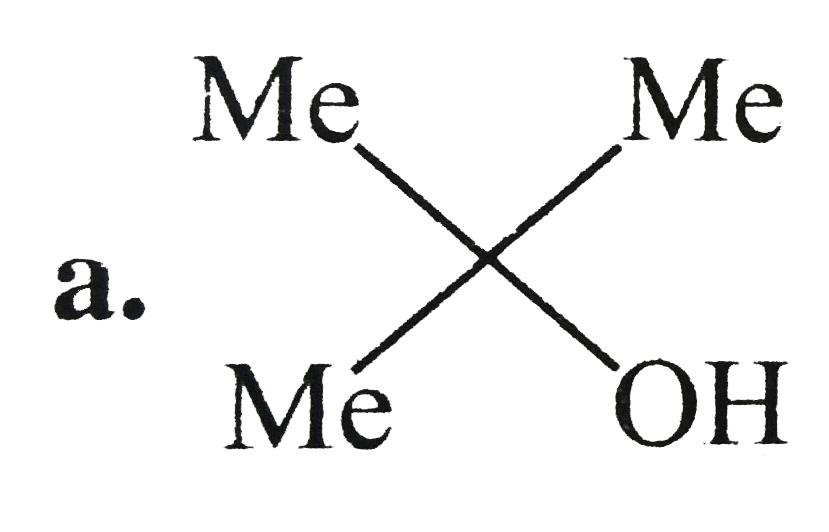
B
C
D
Video Solution
Text Solution
The correct Answer is:A
(a) 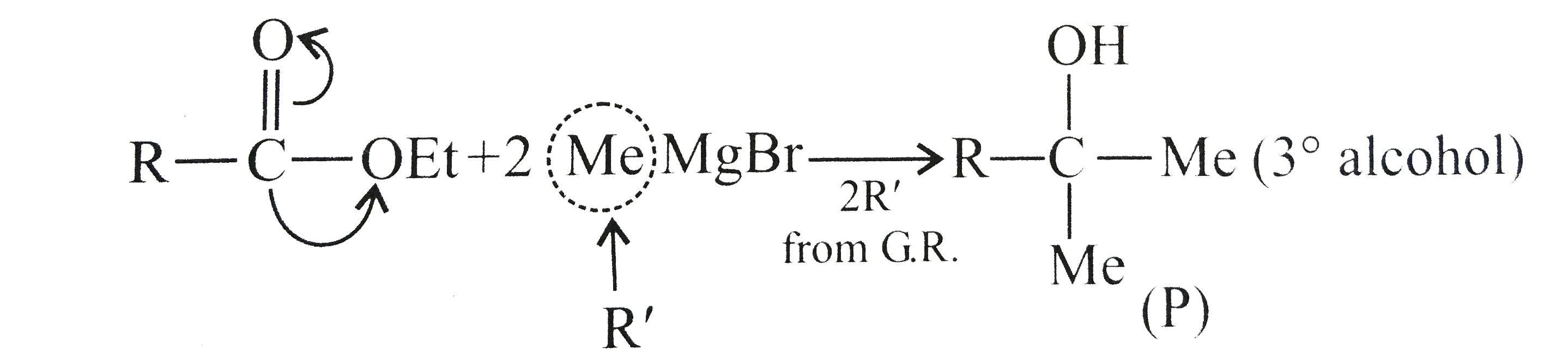
The product (P) should at least two Me groups. Only (a) contains at least two Me groups. In (a), there are three Me groups, so one Me groups has come from the ester. Therefore, the structure of the ester is ⎛⎜
⎜⎝Me−O∣∣C−OEt⎞⎟
⎟⎠ (Ethyl acetate or ethyl ethanoate).
|
Similar Questions
Knowledge Check
Ethyl easter CH3MgBr−−−−−→excessP, the product P is
A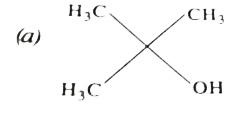 B
B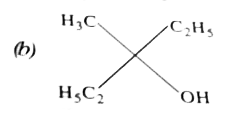 C
C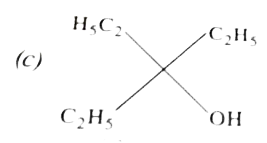 D
D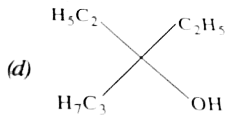
CH3COOC2H5CH3MgBr−−−−−→excessP. The product P will be
A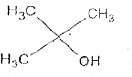 B
B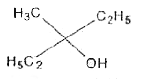 C
C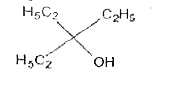 D
D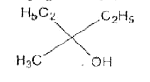
Ethyl ester CH3MgBr−−−−−→excessP. The product P is
A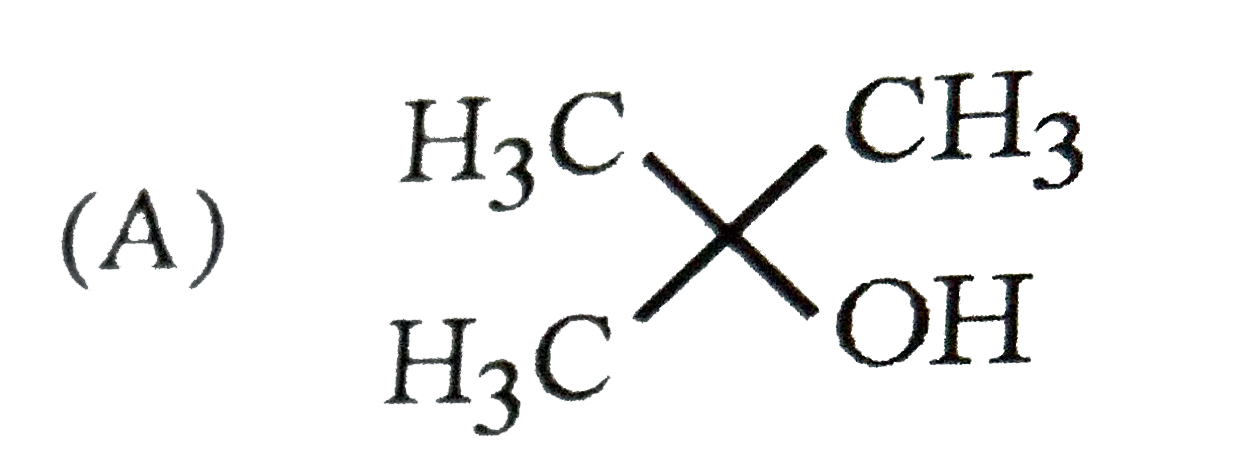 B
B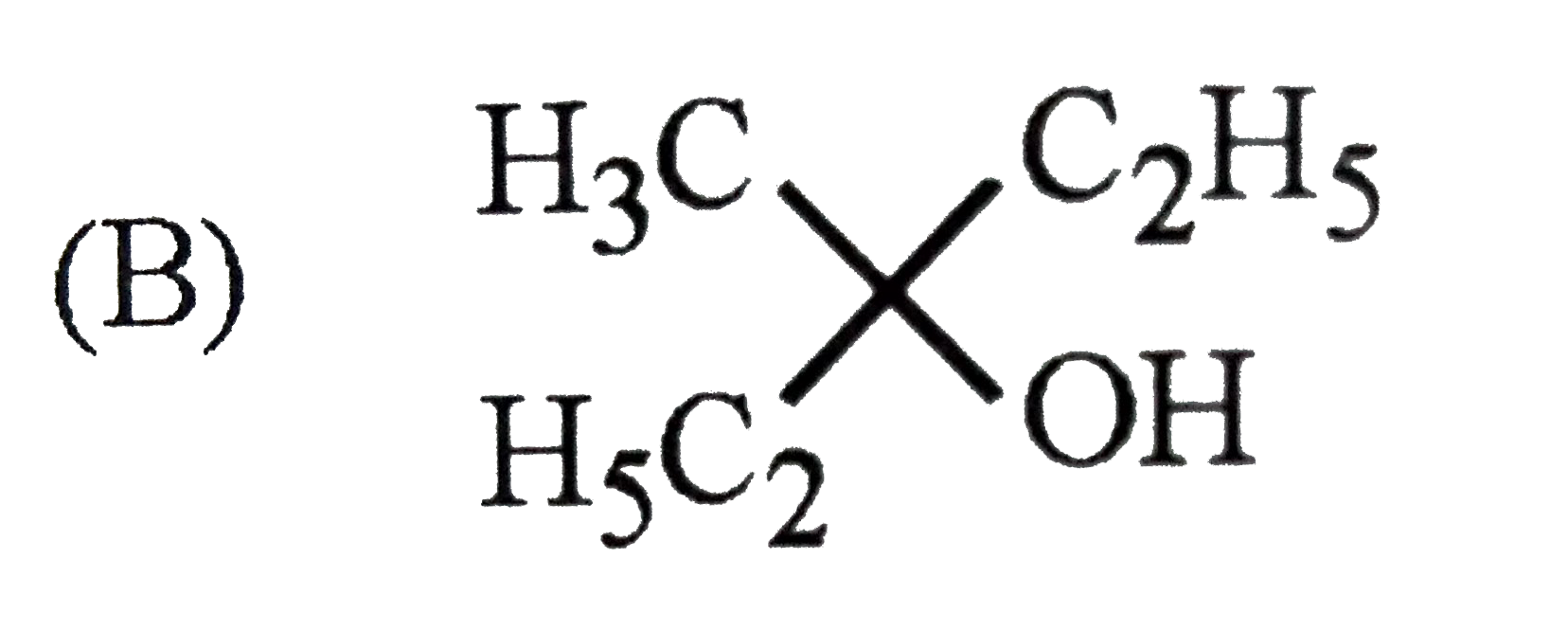 C
C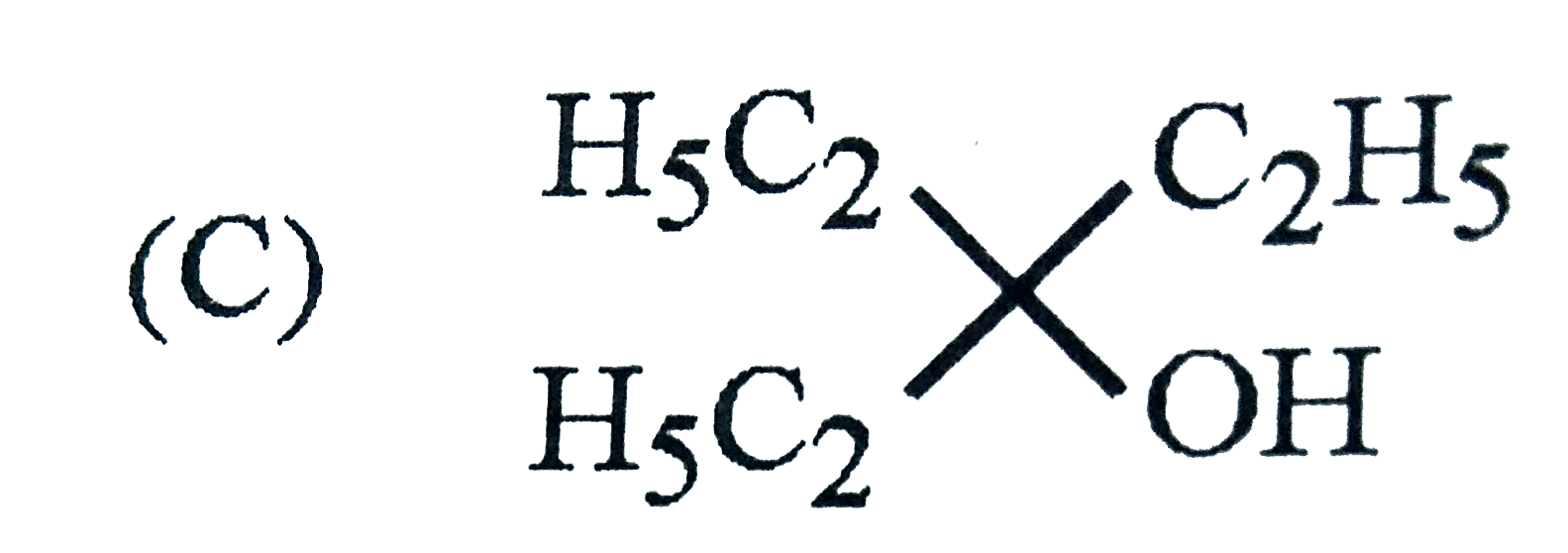 D
D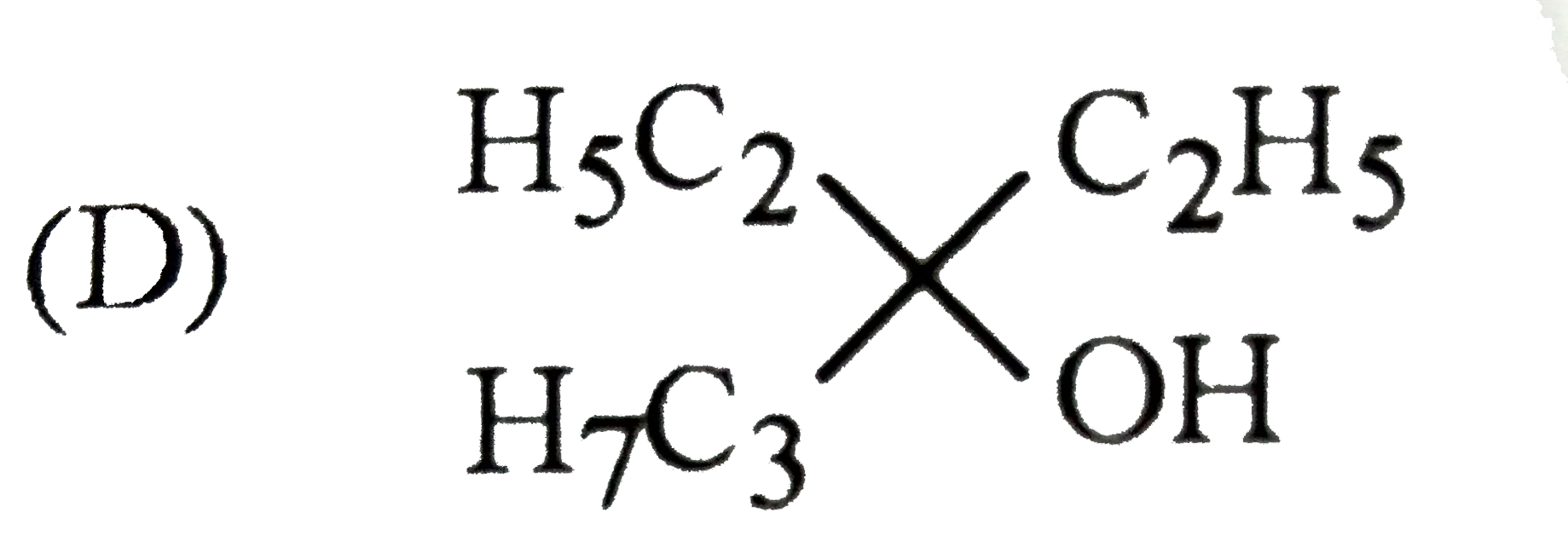
Ethyl acetate CH3MgBr−−−−−→ExcessH2O+−−−→P
The product P will be :-
View SolutionEthylester CH3CH2MgBr−−−−−−−−→excessP. The product P will be
View Solution1−Methylethylene oxideexcess−−→HBrX, Product 'X' will be-
View Solution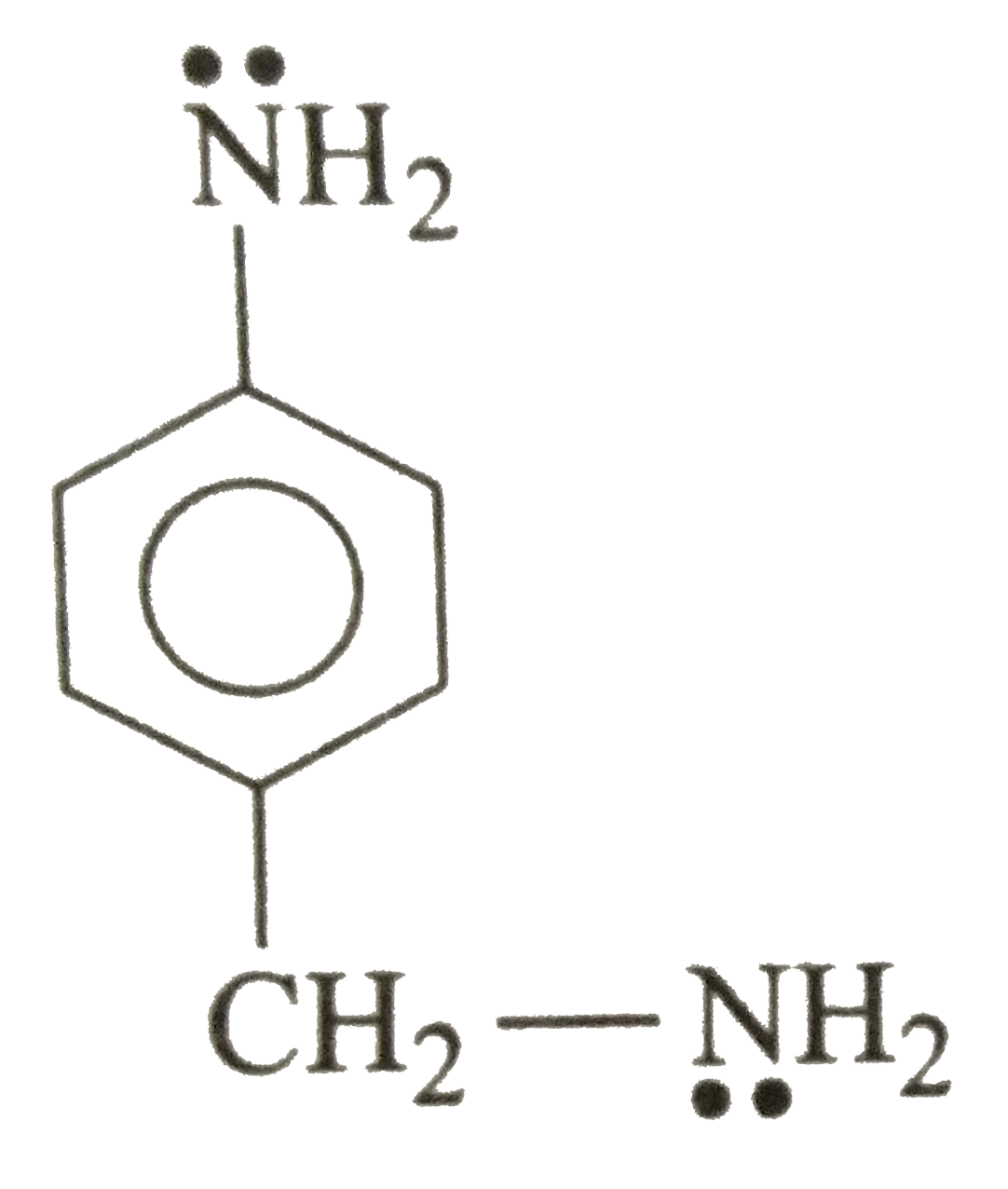 HCI−−−−→(1Mole) Product
HCI−−−−→(1Mole) Product
View SolutionIn the following reaction:
RMgBr+HC(OEt)3Ether−−→H3O+−−−→P
The product 'P' is
View Solution

