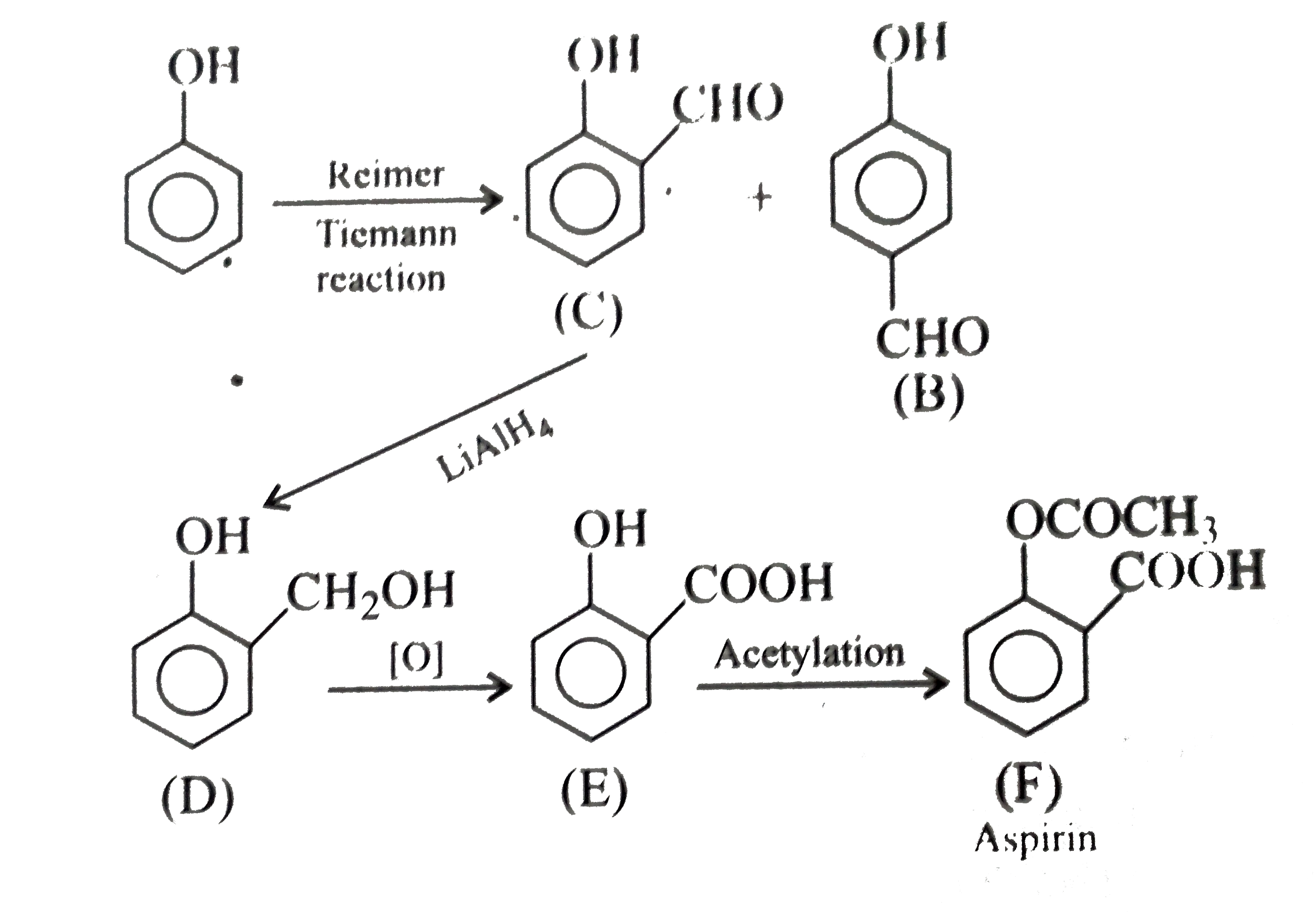
An organic compound (A) gives opsitive Liebermann reaction and on treatment with CHCI3/KOH followed by hydrolysis gives (B) and (C). Compound (B) gives colour with Schiff's reagent but not (C), which is steam volatile. (C ) on treatment with LiAIH4 gives (D), C7H8O2, which on oxidation gives (E). Compound (E) reacts with (CH3CO)2)∅CH3COOH to give a pain reliever (F). Give the structures of (A) to (F) with proper reasoning.
Reactions:
Similar Questions
An organic compound (A) gives positive Liebermann's nitroso reaction and on treatment with CHCl2/KOH followed by hydrolysis gives (B) and (C). Compound (B) gives pink colour with Schiff's reagent but not (C) which is steam volatile. (C) on treatment with LiAlH4 gives (D) (C7H5O2) which on oxidation gives E. 'E' reacts with acetic anhydride and gives compound F. calculate molecular weight of 'F'.
View SolutionAn organic compound (A) reacts with ethanol to give (B) and (C). On acid hydrolysis (C ) yields (B) and (D). oxidation of (D) gives (B). (B) is an acid and forms a salt with Ca(OH)2 which on dry distillation gives (E ), C3H6O. Give the structures of (A) to (E ) with proper reasoning.
View Solution
Knowledge Check
An organic compound ( A ) on reduction gives compound (B) on treatment with CHCI3 and alcoholic KOH gives (C ) on catalytic reduction gives N- Methyl aniline .The compound (A) is
AMethylamineBAnilineCNitrobenzeneDNitro methaneCompound A on reaction with HCN andon further hydrolysis gives
C2H5−OH∣C∣CH3−COOH
Hence the compound A is:AethanolBpropanalCpropanoneDbutanoneAn organic compound (A) on reduction gives compound (B). (B) on treatment with CHCl3 and alcoholic KOH gives ( C). ( C) on catalytic reduction gives N-methylaniline. The compound (A) is
AmethylamineBnitromethaneCanilineDnitrobenzene
An organic compound A(C6H6O) gives dark green colouration with ferric chloride. On treatment with CHCl3andKOH, followed by acidification gives compound B. Compound B can also be obtained from compound C on reaction with pyridinium chlorochromate (PCC). Identify A, B and C
View SolutionTwo isomeric mononitro derivaters (B) and (C) are obtained by the nirtration of an organic compound (A),C7H8O. Treatment of (A0 with acetyl chloride products (D) which on reaction with CrO2Cl2 gives (E) whose oxidation with neutral KMnO4 followed by acidification gives (F). Compound (F) on heating gives pheonl. (A) on treatment with alkaline C6H5SO2Cl produces. (A) on treatment with alkaline C6H5SO2Cl produces (G) which on oxidiation with KMnO4 gives (H). Hydrolysis of (H) also gives (F). Give teh structures of (A) to (H).
View SolutionCompound (A) C8H6O2 on treatment with aq. NaOH followed by acidification gives (B) C8H8O3 which on oxidation gives benzoice acid only :
View SolutionAn compound (A ) C4H8Cl2 on alkaline hydrolysis to give compound (B ) C4H8O which gives an oxime and positive Tollen's reagent test . What is the structure of (A) ?
View SolutionAn organic compound (A) on reduction gives compound (B) which on treatment with CHCI3 and alcoholic KOH gives compound (C) which on hydrolysis gives aniline but on compound (C) which on hydrolysis gives aniline but on catalytic reduction gives n-methylaniline The compound (A) is .
View Solution
