Which of the following statements is/are correct about the given reaction ?
(A) 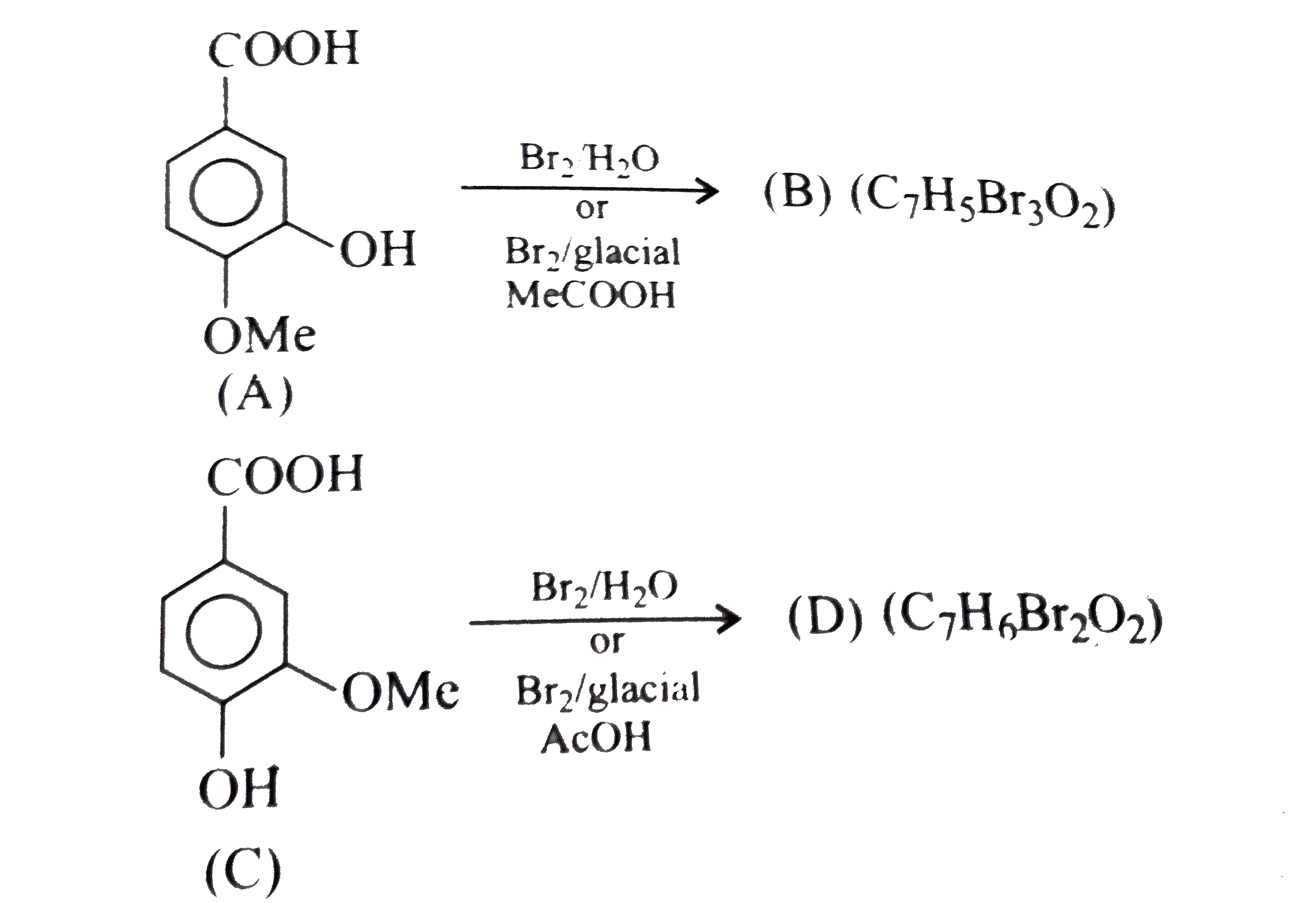 (B) (C7H5Br3O2)
(B) (C7H5Br3O2)
(C )  (D) (C7H6Br2O2)
(D) (C7H6Br2O2)
The products (B) and (D), respectively, are:
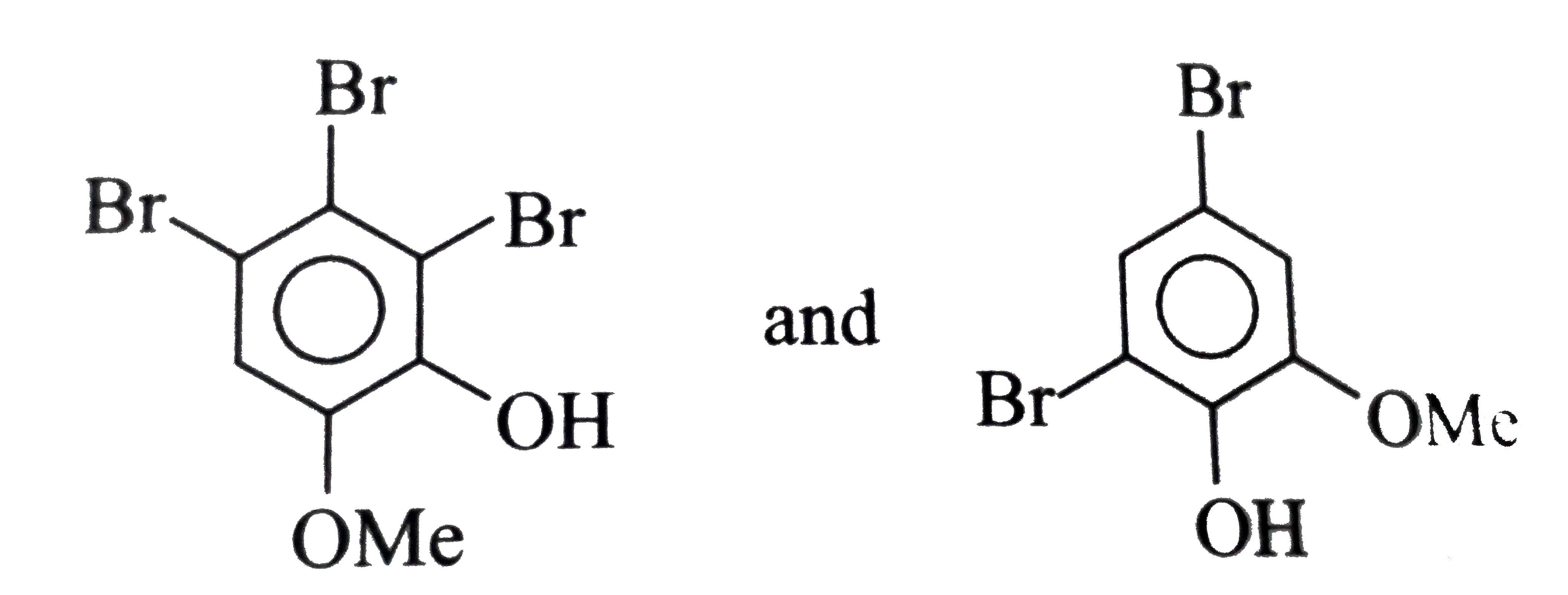
The products (B) and (D), respectivvely, are:
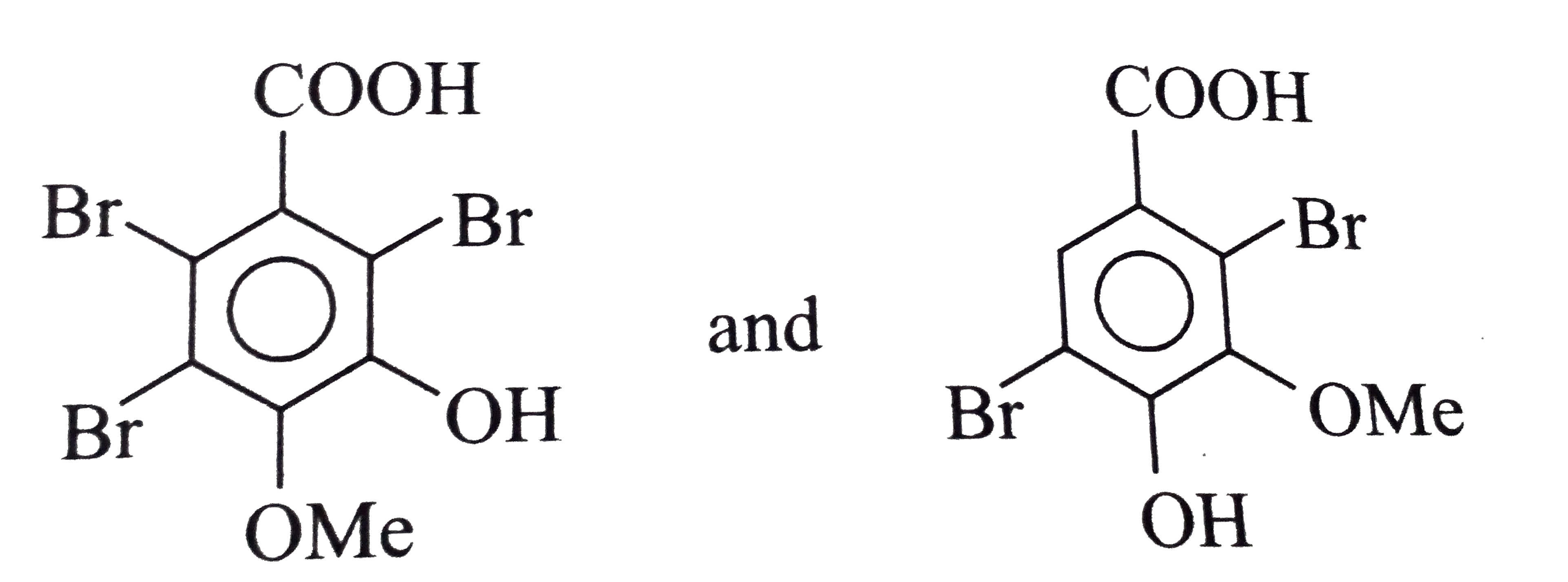
In the formation of the both products (B) and (D), one Be is obtained by ipso substitution while the other Br is obtained by SE reaction.
In the formation of (B) and (D), two Br atoms are obtained by ispo substitution, whereas the other Br is obtained by SE reaction.
The correct Answer is:A, C
NA
Similar Questions
An organic compound (A) C_(5)H_(7)OC l reacts rapidly with ethanol to give (B) C_(7)H_(12)O_(2) . (A) also reacts with water to produce acid which reacs with Br_(2) to give C_(5)H_(8)Br_(2)O_(2) . 'B' on boiling with aqueous H_(2)SO_(4) formed an acid (C). when (C) is oxidized with KMnO_(4) to an acid (D) C_(4)H_(6)O_(3) and CO_(2) gas are produced. on mild heating, 'D' gives E. E cannot reduce Tollen's reagent. calculate the molecular weight of E.
View Solution(b) A aromatic hydrocarbon (A) of molecular formula C_(6)H_(6) reacts with Conc. HNO_(3) and Conc. H_(2)SO_(4) gives (B) of formula C_(6)H_(5)O_(2)N . (B) on reaction with Sn//HCl gives (C ) of formula C_(6)H_(7)N which answers carbylamine reaction. (C ) on treatment with chloroform and alkali gives (D ) of formula C_(7)H_(5)N . Identify A, B, C, D and explain the reactions involved.
View SolutionA aromatic hydrocarbon (A) of molecular formula C_(6)H_(6) reacts with Con HNO_(3) and Conc. H_(2)SO_(4) gives (B) of formula C_(6)H_(5)O_(2)N . (B) on reaction with Sn/HCl gives (C) formula C_(6)H_(7)N which answers carbylamine reaction. (C) on treatment with chloroform and alkalli gives (D) of formula C_(7)H_(5)N . Identify A,B,C,D and explain the reactions involved.
View Solution…. Is the name of C6H4Br2
View Solution…. Is the name of C6H4Br2
View SolutionAn organic compound (A) (C_(5)H_(7)OCl) reacts rapidly with ethanol to give (B) (C_(7)H_(12)O_(2)) . (A) also reacts with water to produce an acid which reacts with bromine to give (C ) (C_(5)H_(8)Br_(2)O_(2)) . (B) on boiling with H_(2)SO_(4) forms an acid (D). When (D) is oxidised with KMnO_(4) an acid (E ) (C_(4)H_(6)O_(3)) is produced. On mild heating, (E ) gives (F) (C_(3)H_(6)O) which cannot be oxidised by ammoniacal AgNO_(3) . Identify the compounds (A) to (F).
View SolutionBalance : C6H5CHO+Cr2O2−7→C6H5COOH+Cr3+
View SolutionConsider the following sequence of reactions
[A](C3H9N)NaNO2/HCl−−−−−−−→[B](C3H8O)K2Cr2O7−−−−−→H2SO4(C3H6O2)[C]
The compound [A] is
View Solution

