Which among the following compounds will not give effervescence with sodium bicarbonate ?
A
C6H5CO2H
B
C6H5SO3H
C
C6H5OH
D
Picric acid
Video Solution
Text Solution
The correct Answer is:C
Phenol does not react with NaHCO3 or NaCO3. Although picric acid is a phenolic compound (2,4,6-trinitrophenol), due to the presence of 3NaO2 groups (EWG), it is highly acidic and reacts with NaHCO3 or NaCO3.
|
Similar Questions
Knowledge Check
One among the following compounds will not give effevesecence with sodium carbonate :
AC6H5CO2HB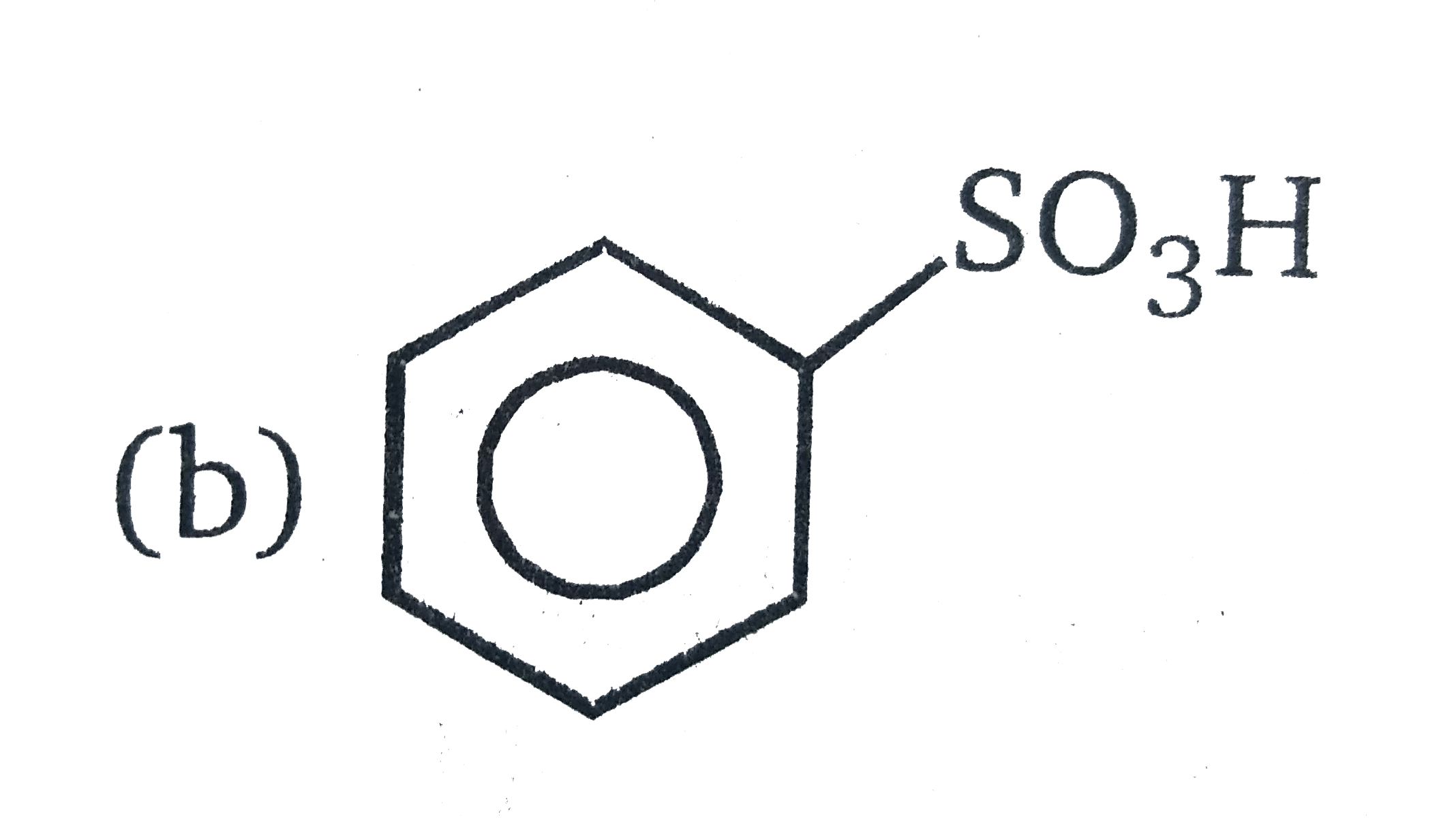 CC6H5OHD
CC6H5OHD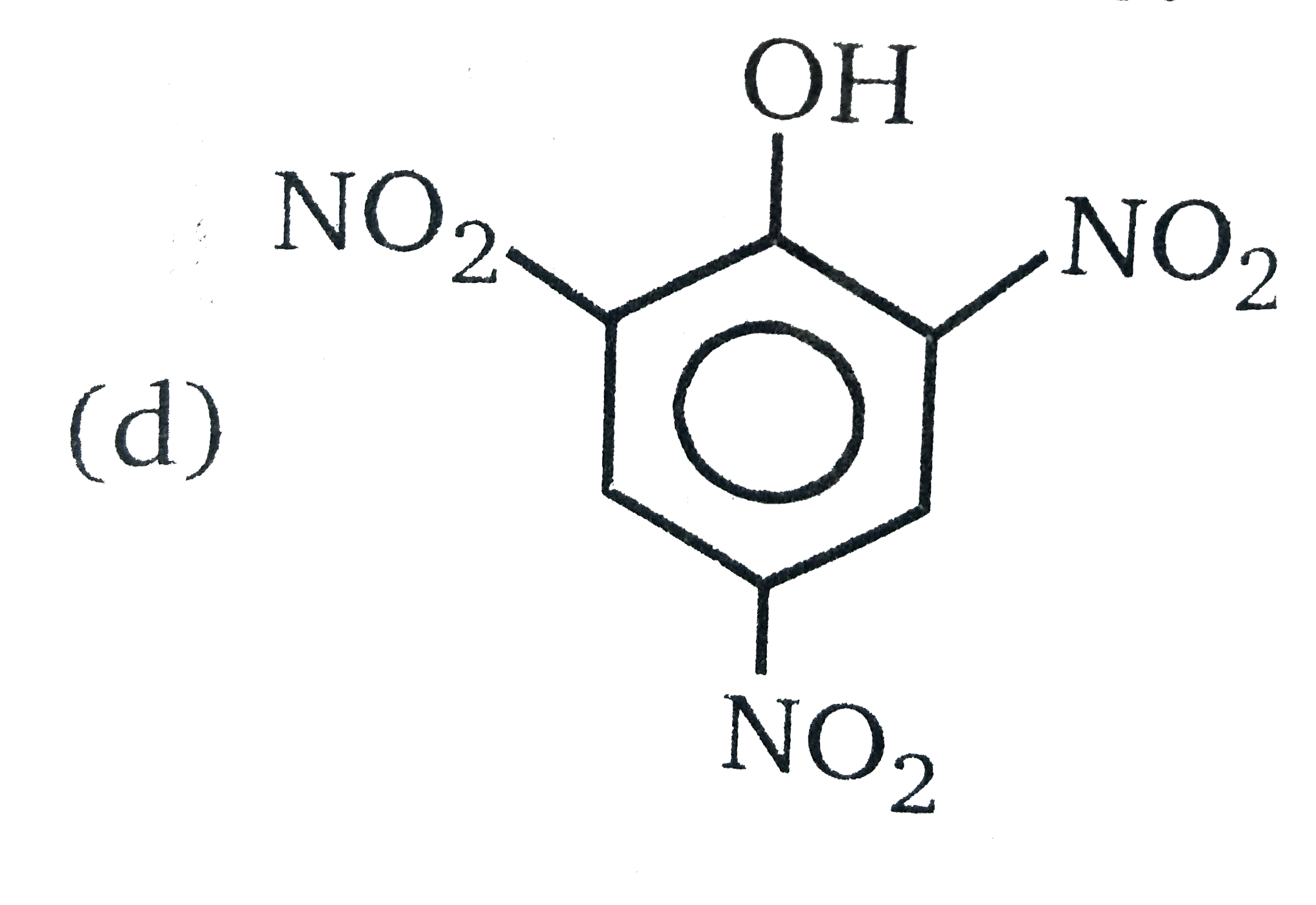
which of the following amine will not give effervescence with HNO2
ACH3NH2BC2H5NH2C(CH3)2CHNH2D(CH3)2NHSodium bicarbonate has :
AIonic bondBCovalent bondCHydrogen bondDAll of these
Which of the following would produce effervescence with sodium bicarbonate ?
View SolutionWhich of the following would produce effervescence with sodium bicarbonate ?
View SolutionWhich of the following compounds will not be soluble in sodium bicarbonate ?
View SolutionWhich of the following would produce effervesence with sodium bicarbonate ?
View SolutionWhich of the following does not give effervescence with NaHCO3 ?
View Solution

