Two moles of HCHO and 1 mol of PhCHO react with conc. NaOH. What are the produsts quantitatively?
Video Solution
Text Solution
Initially there will be crossed Cannizzaro reaction between HCHO and PhCHO.
HCHO+PhCHONaOH−−−→HCOONa1mol+PhCH2OH1mol
HCHO(12)mol+HCHO(12)molNaOH−−−→HCOONa(12)mol+CH3OH(12)mol
Remaining 1 mol of HCHO will give Cannizzaro reaction. So 1/2 mol of HCHO will react with 1/2 mol of HCHO to give 1/2 mol of HCOONa and 1/2 mol of CH3OH. Hence, 1.5 mol of HCOONa, 1 mol of PhCH2OH, and 1/2 mol of CH3OH are formed.
|
Similar Questions
Methyl cyclohexene on reductive oxonzolysis followed by reaction with dil. NaOH gives [X] number of products A mixture of HCHO + PhCHO on treatment with conc. NaOH gives Y number of products 1,2-Dimethylcyclohexene on treatment with metachloroperbenzoic acids give (Z) number of products .
View Solution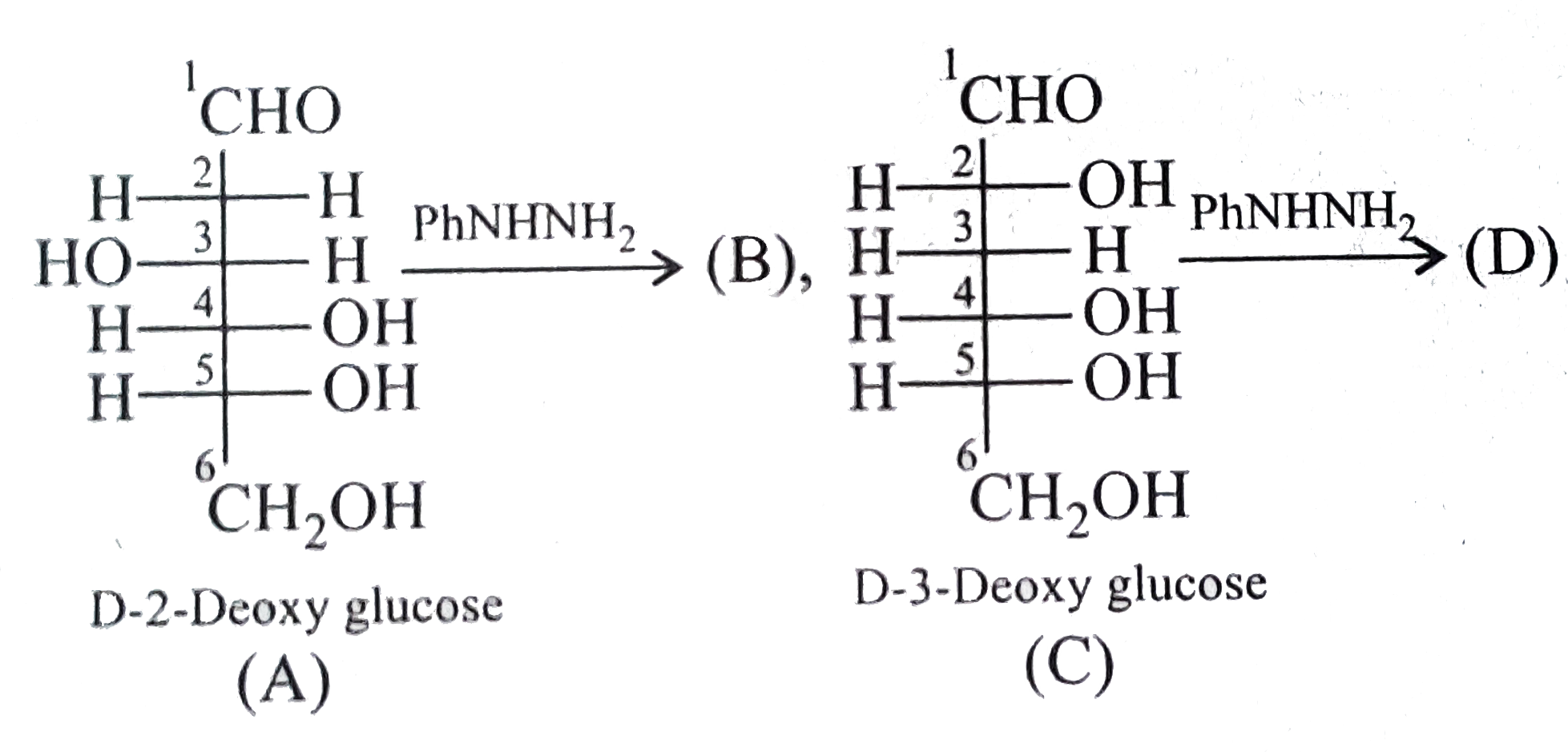
How manty moles of (C) react with 1molPhNHNH2
View Solution
Knowledge Check
Two moles of HCHO and 1 mol of PhCHO react with conc. NaOH. What are the products quantitatively?
A1 mole of PhCH2OH, 2 mol of CH3OH, and I mol of HCOONa.B1 mol of PhCH2OH, 1.5 mol of CH3OH, and 0.5 mol of HCOONa.C1 mol of PhCH2OH, 0.5 mol of CH3OH, and 1.5 mol of HCOONa.D1.5 mol of PhCH2OH, 1 mol of CH3OH, and 1 mol of HCOONa.Benzenaldehyde reacts with conc. NaOH to give :
Asodium benzoteBbenzyl alcoholCphenolDbenzyl alcohol and sodium benzoate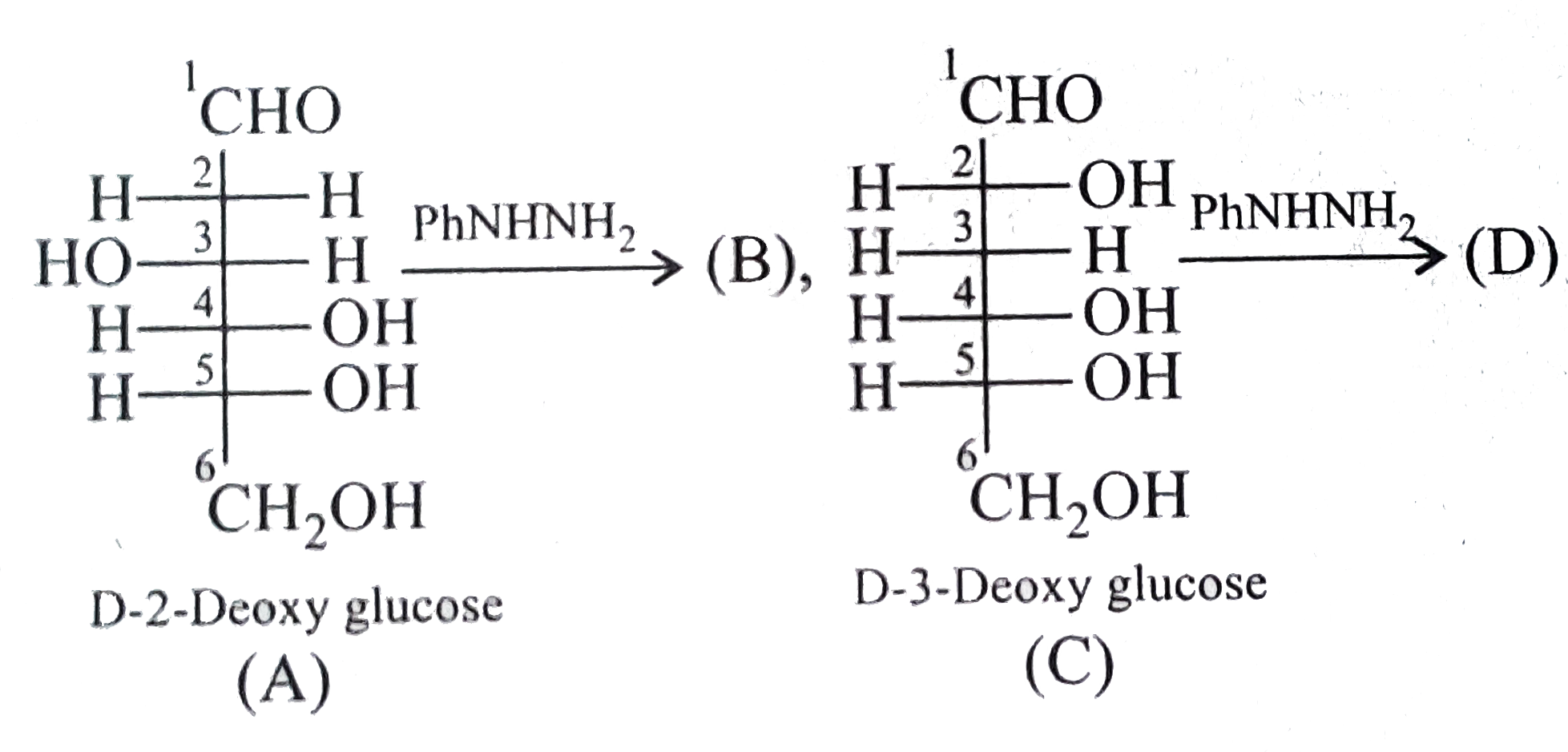
How many moles of PhNHNH2 react with 1mol(A)?A1B2C3D4
Two mole of alkyl iodide is formed when ether react with
View Solution1 mol alcohol reacts with Na to give what weight of hydrogen ?
View SolutionWhat happens when methane reacts with conc. HNO3 at high temperature?
View SolutionAn alkene gives two moles of HCHO, one mole of CO2 and one mole of CH3COCHO on ozonolysis. The structure of alkene is
View Solution1 mole alcohol reacts with Na to give what weight of hydrogen?
View Solution
