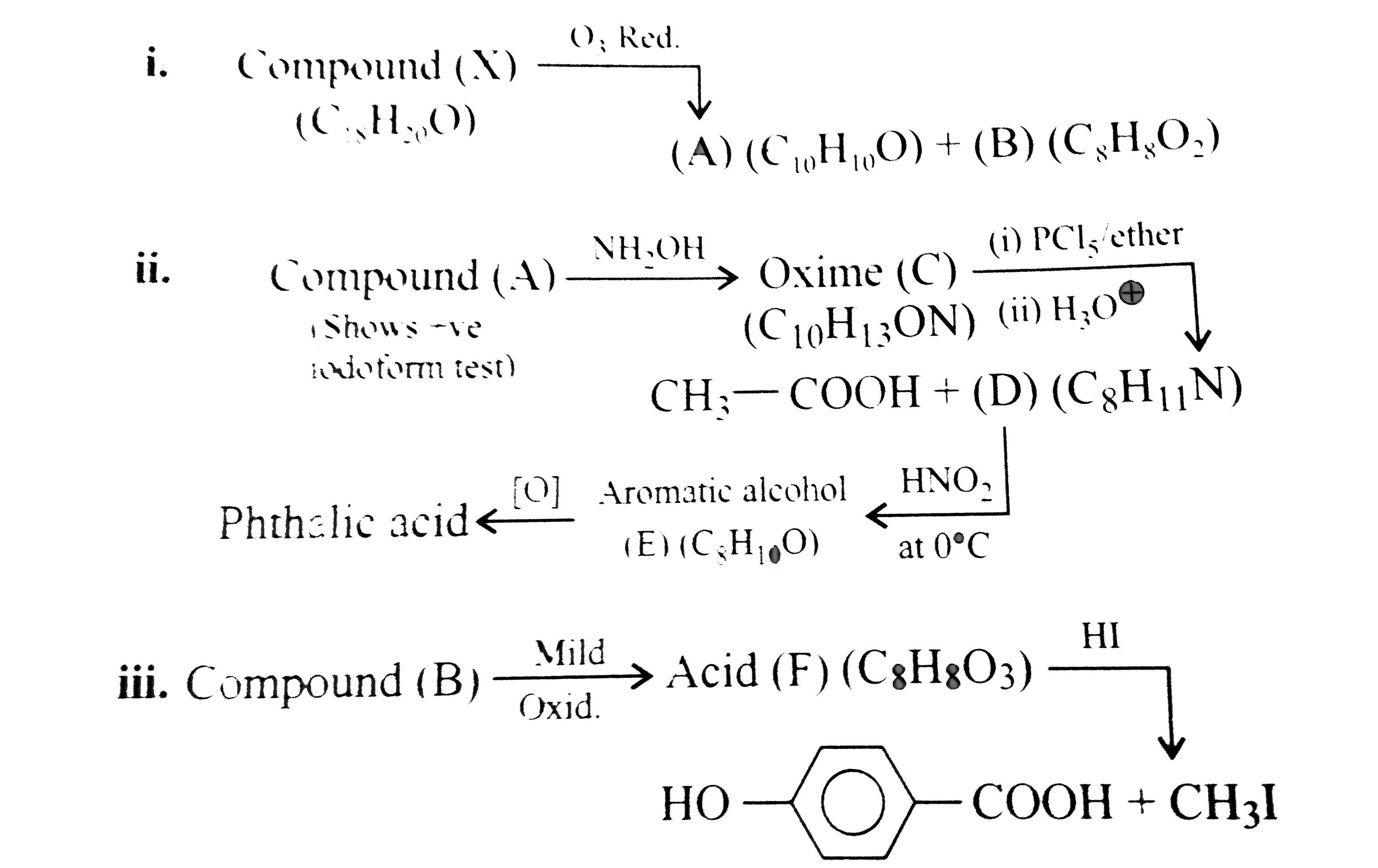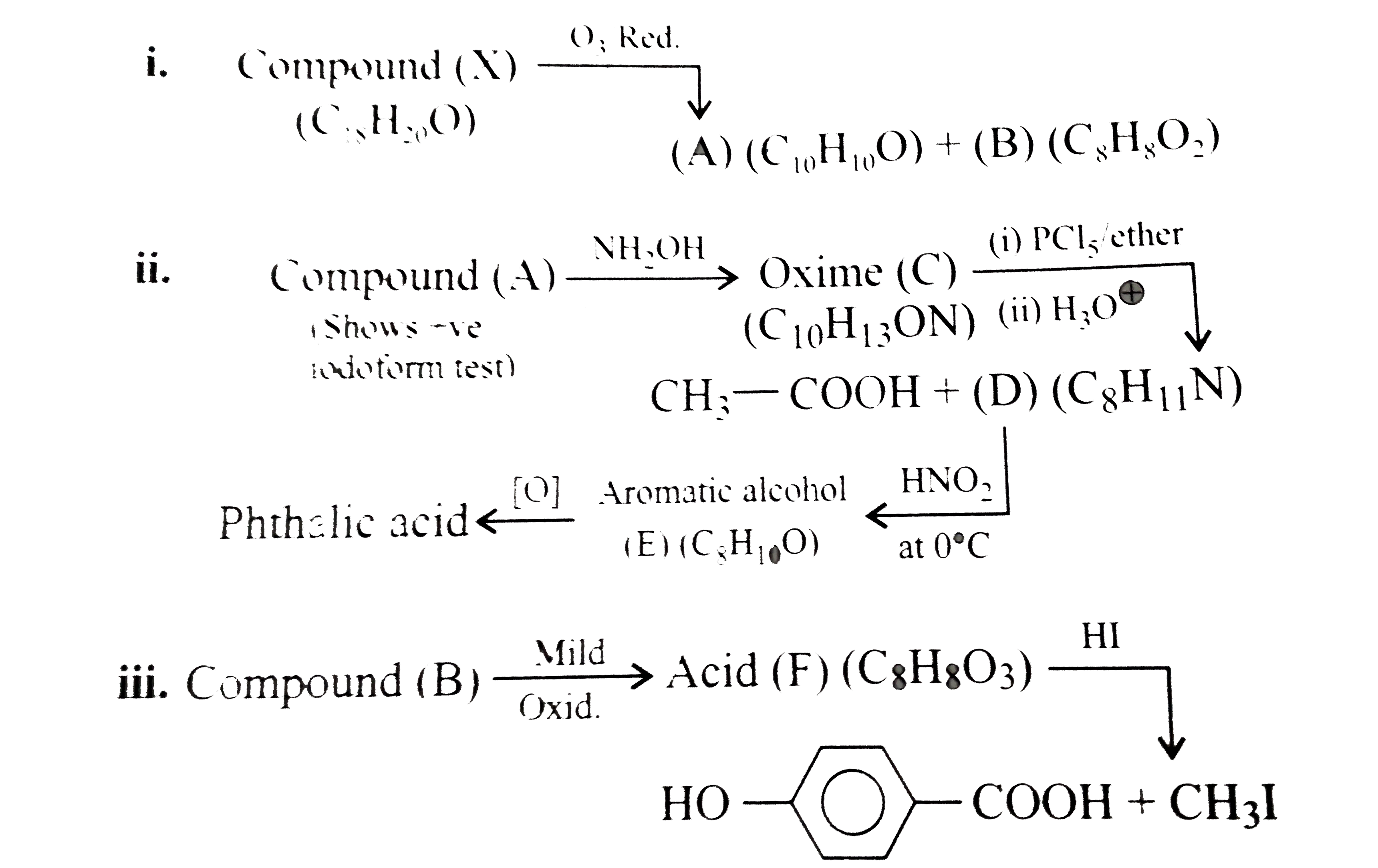i. 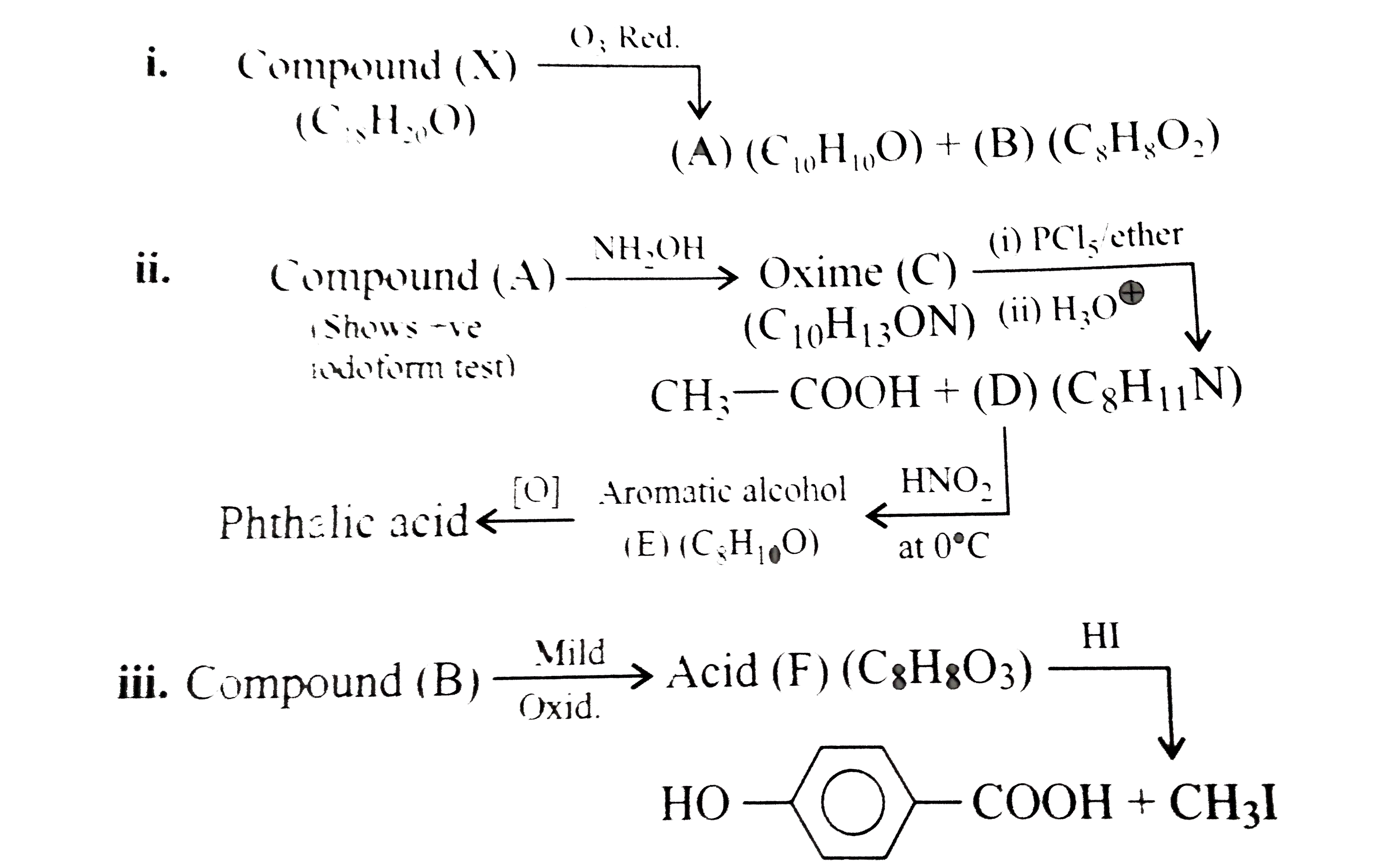
ii.
iv. Compound (X) has the highest melting point among its isomers.
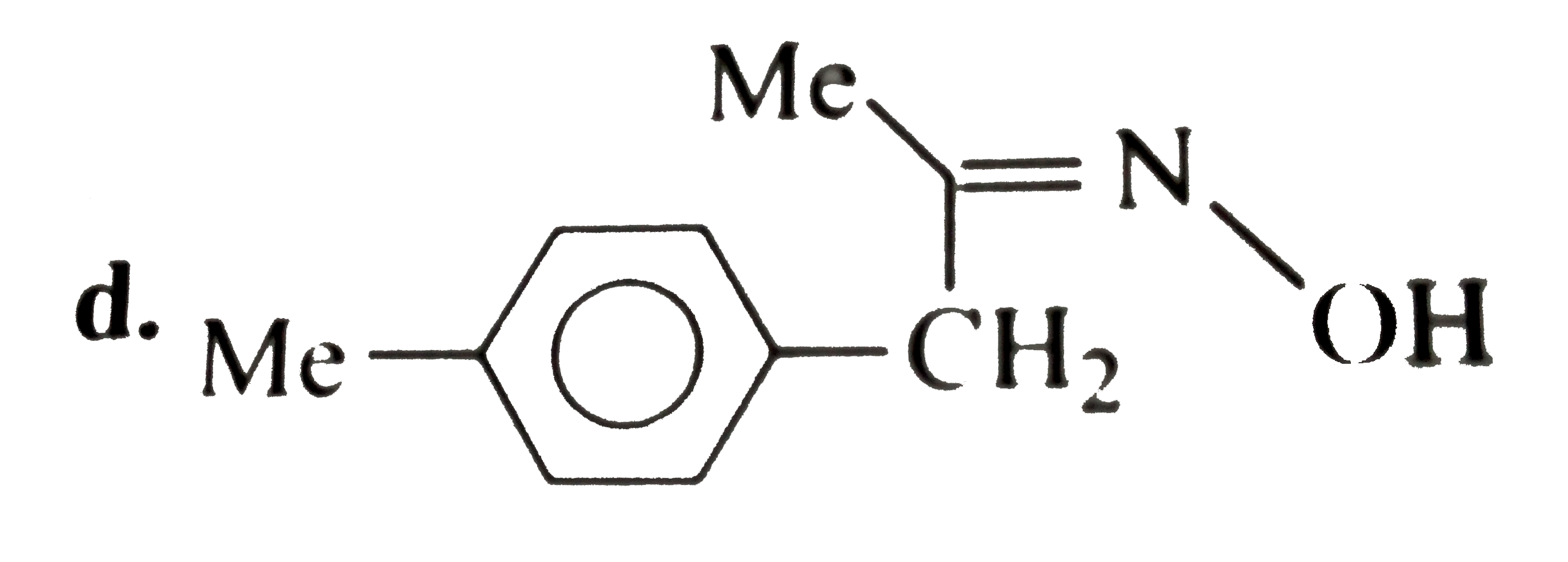
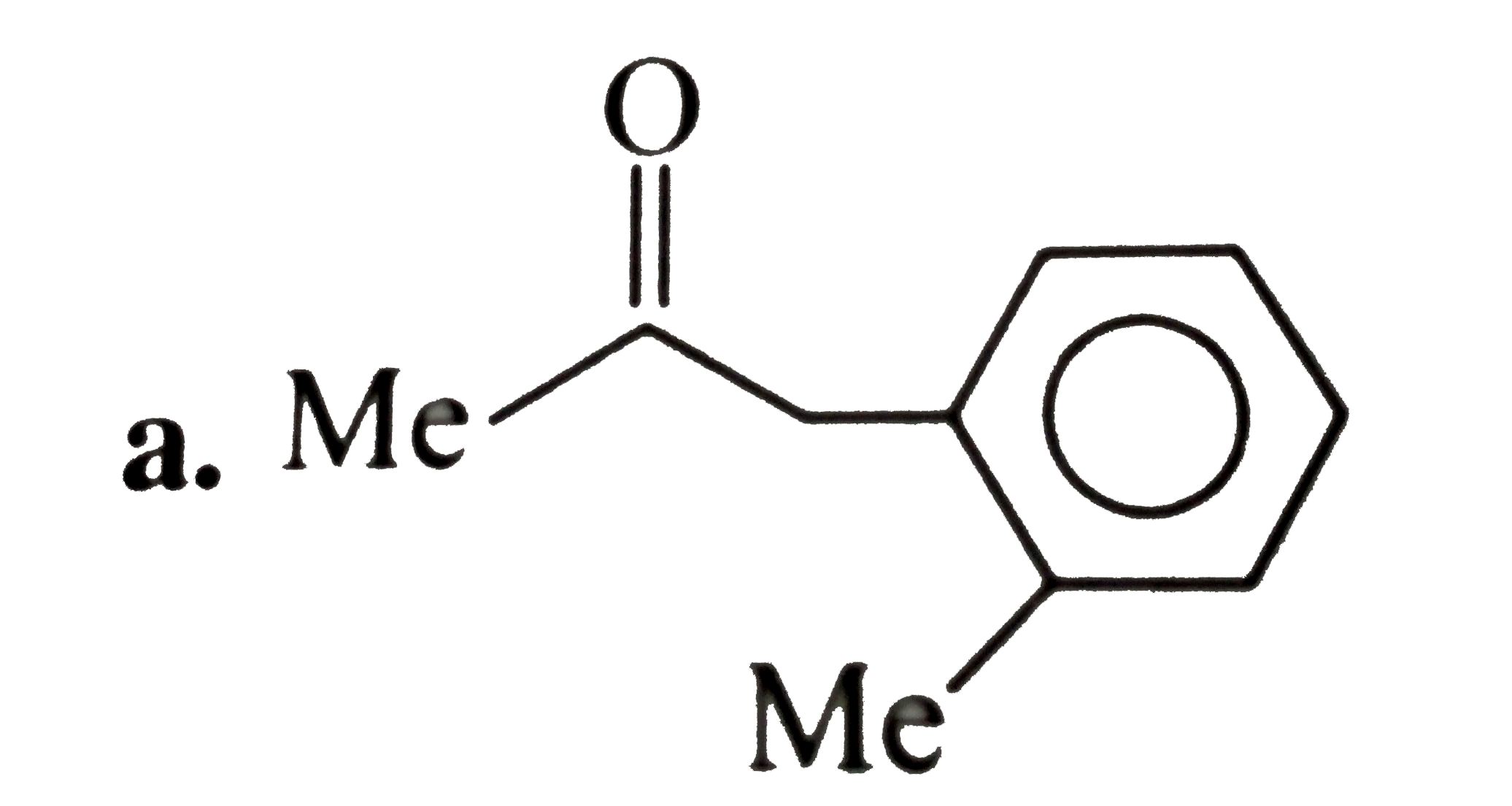
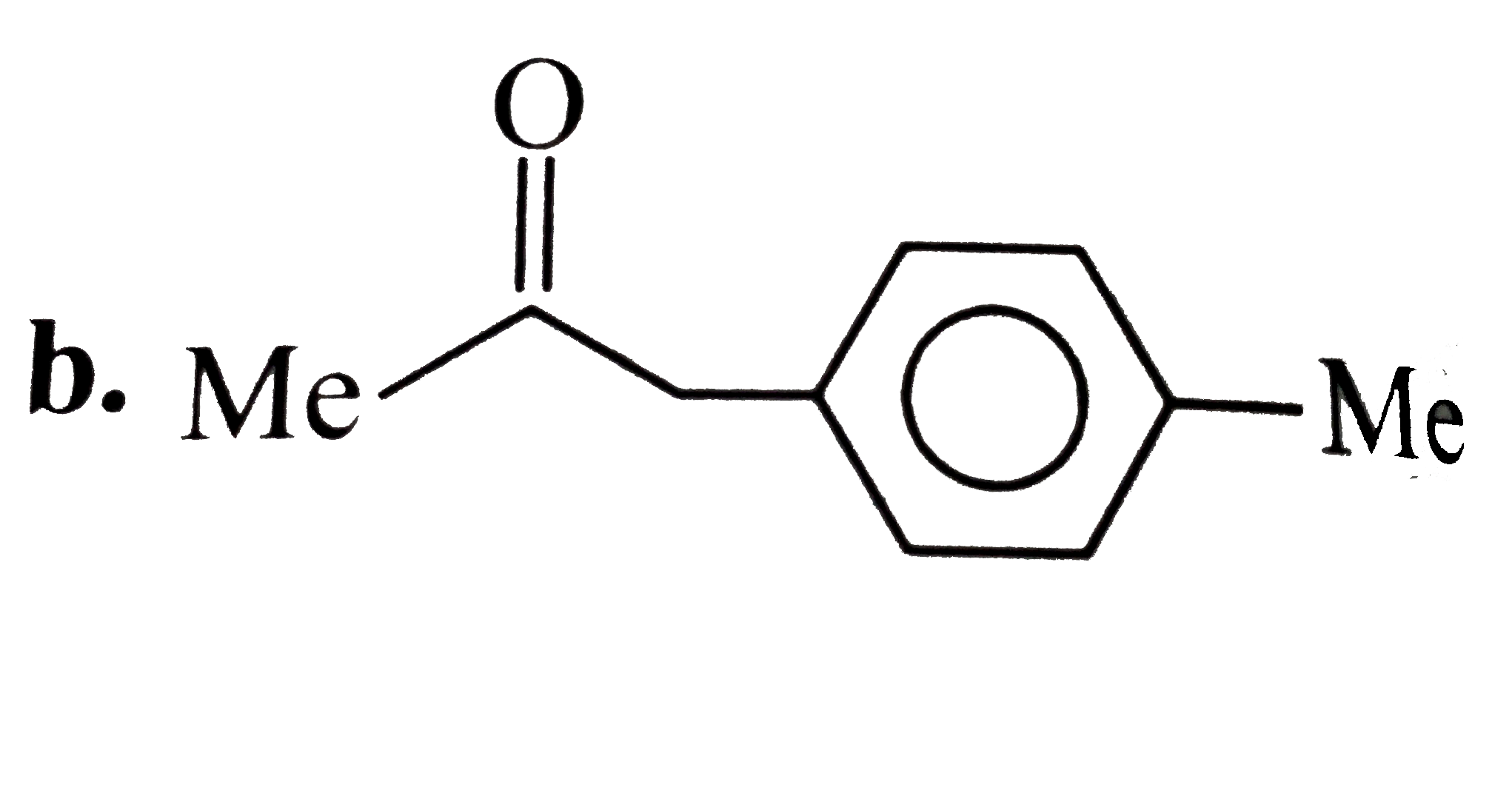
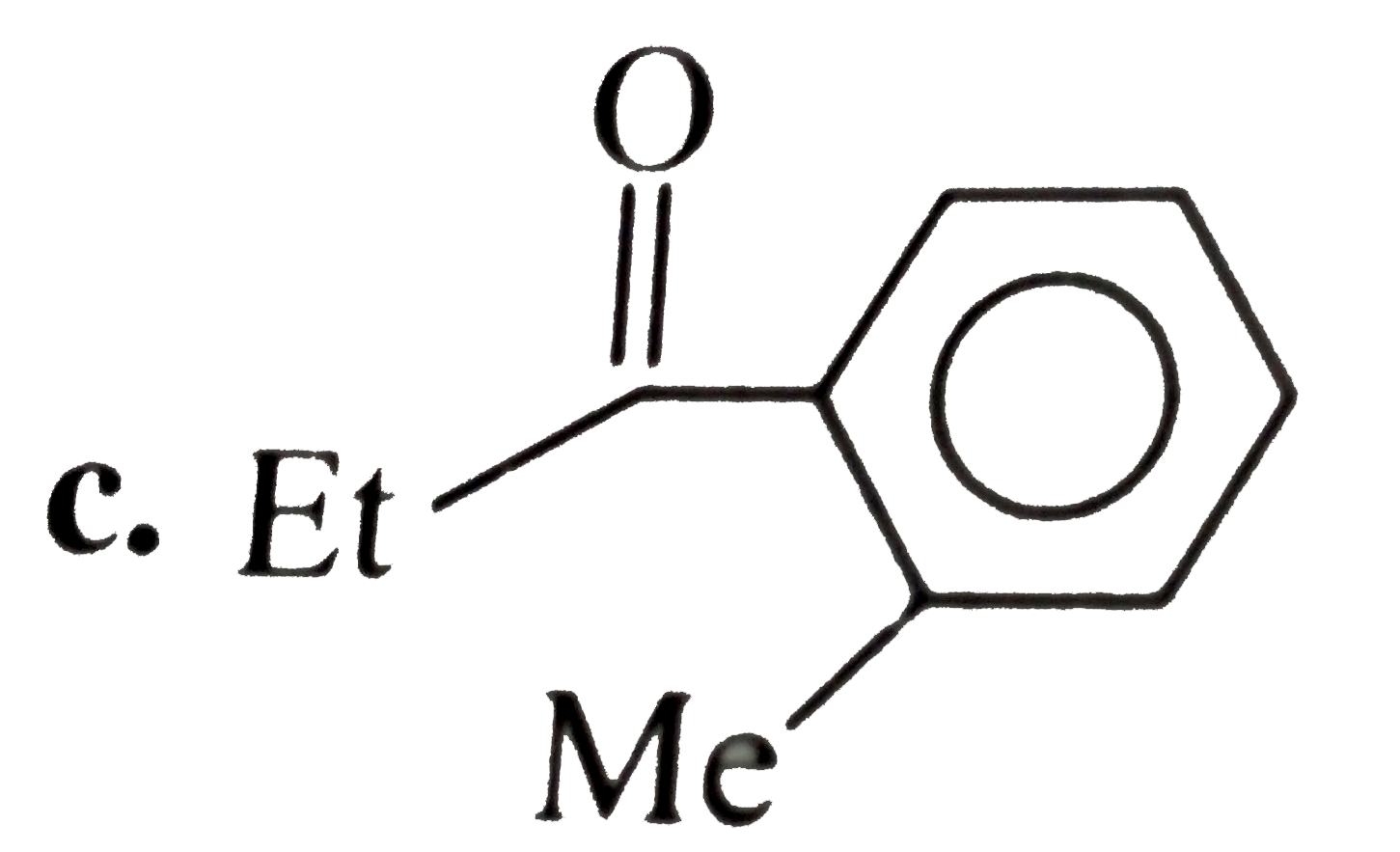
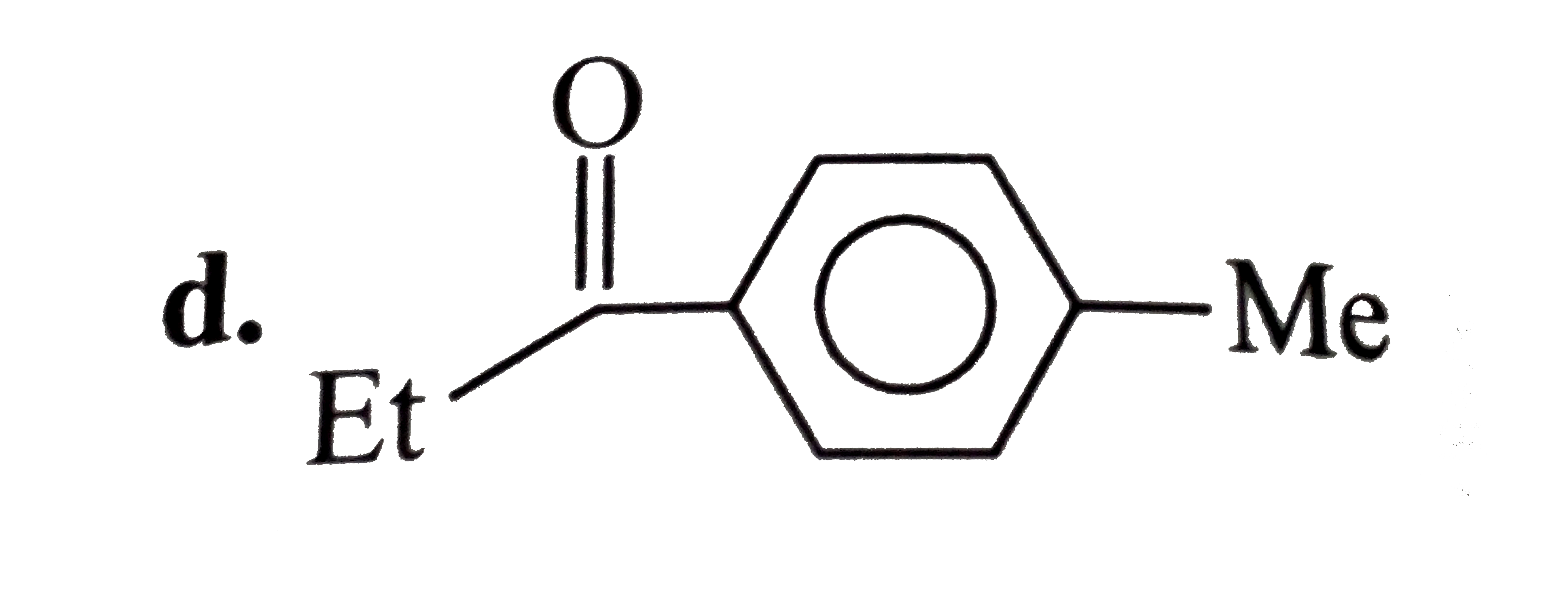
Compound (A) is:
A
B
C
D
Video Solution
Text Solution
The correct Answer is:A
Proceed reverse to find the structure of (B)
i. 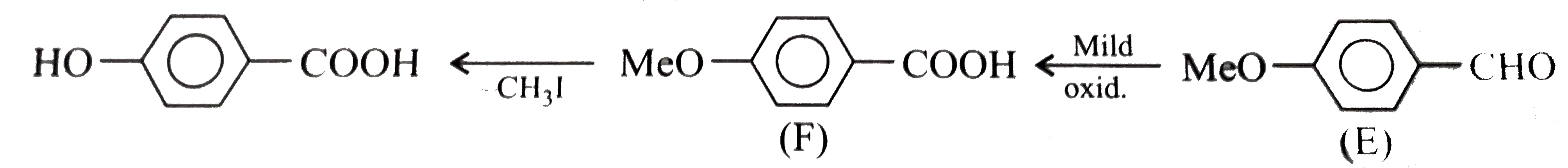
ii. Similarly, proceed reverse to find the structure of (A).
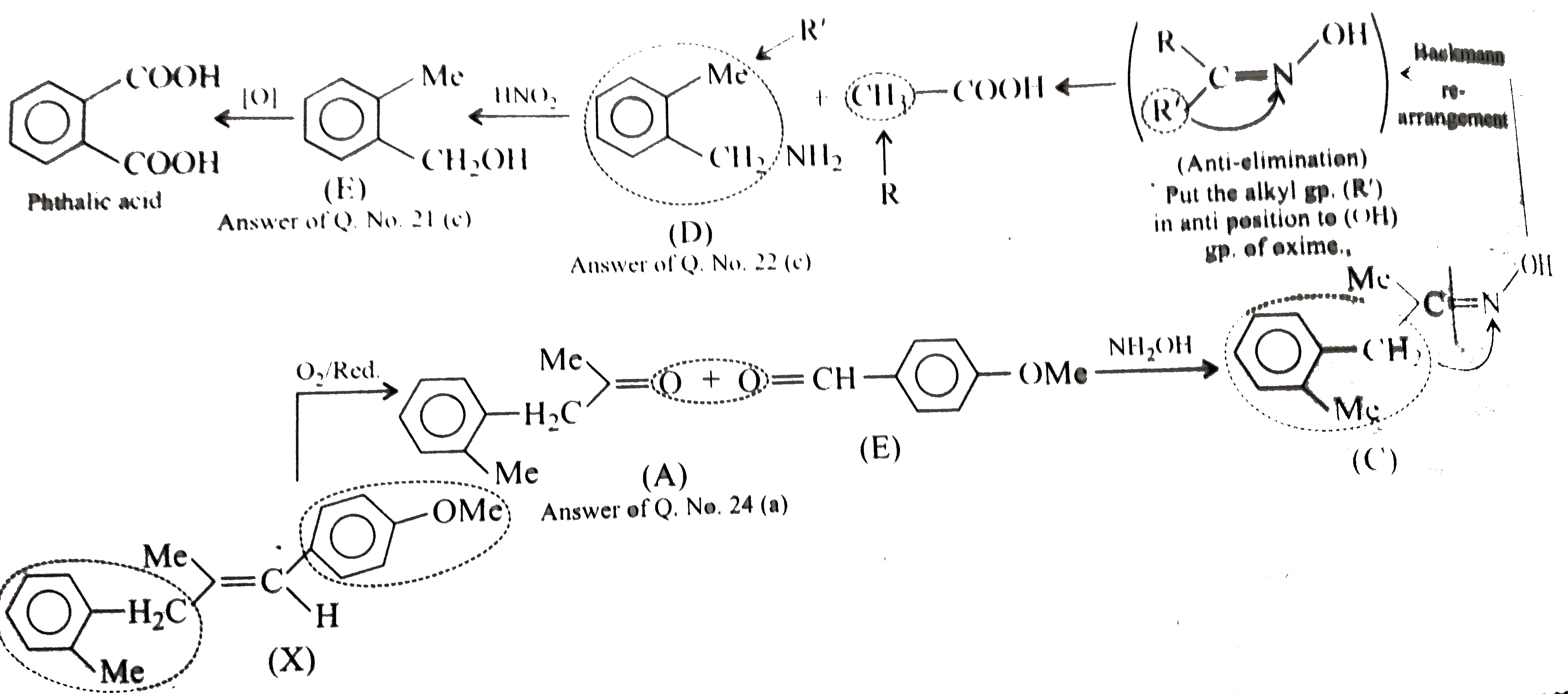
iii. Similarly proceed reverse to get the structure of (X) by joining (A) and (E ) as shown above. (X) is a trans-isomer because its melting point is high.
|
Topper's Solved these Questions
- ALIPHATIC AND AROMATIC ALDEHYDES AND KETONESBook:CENGAGE CHEMISTRYChapter:ALIPHATIC AND AROMATIC ALDEHYDES AND KETONESExercise:Exercises Multiple Correct
Explore 40 Videos
- ALIPHATIC AND AROMATIC ALDEHYDES AND KETONESBook:CENGAGE CHEMISTRYChapter:ALIPHATIC AND AROMATIC ALDEHYDES AND KETONESExercise:Exercises Single Correct
Explore 72 Videos
- ALIPHATIC AND AROMATIC ALDEHYDES AND KETONESBook:CENGAGE CHEMISTRYChapter:ALIPHATIC AND AROMATIC ALDEHYDES AND KETONESExercise:Exercises Concept Application
Explore 20 Videos
- ALCOHOL,PHENOL AND ETHERSBook:CENGAGE CHEMISTRYChapter:ALCOHOL,PHENOL AND ETHERSExercise:Archives Analytical And Descriptive
Explore 15 Videos
- AMINESBook:CENGAGE CHEMISTRYChapter:AMINESExercise:QUESTION BANK
Explore 1 Video
Similar Questions
Knowledge Check
i.
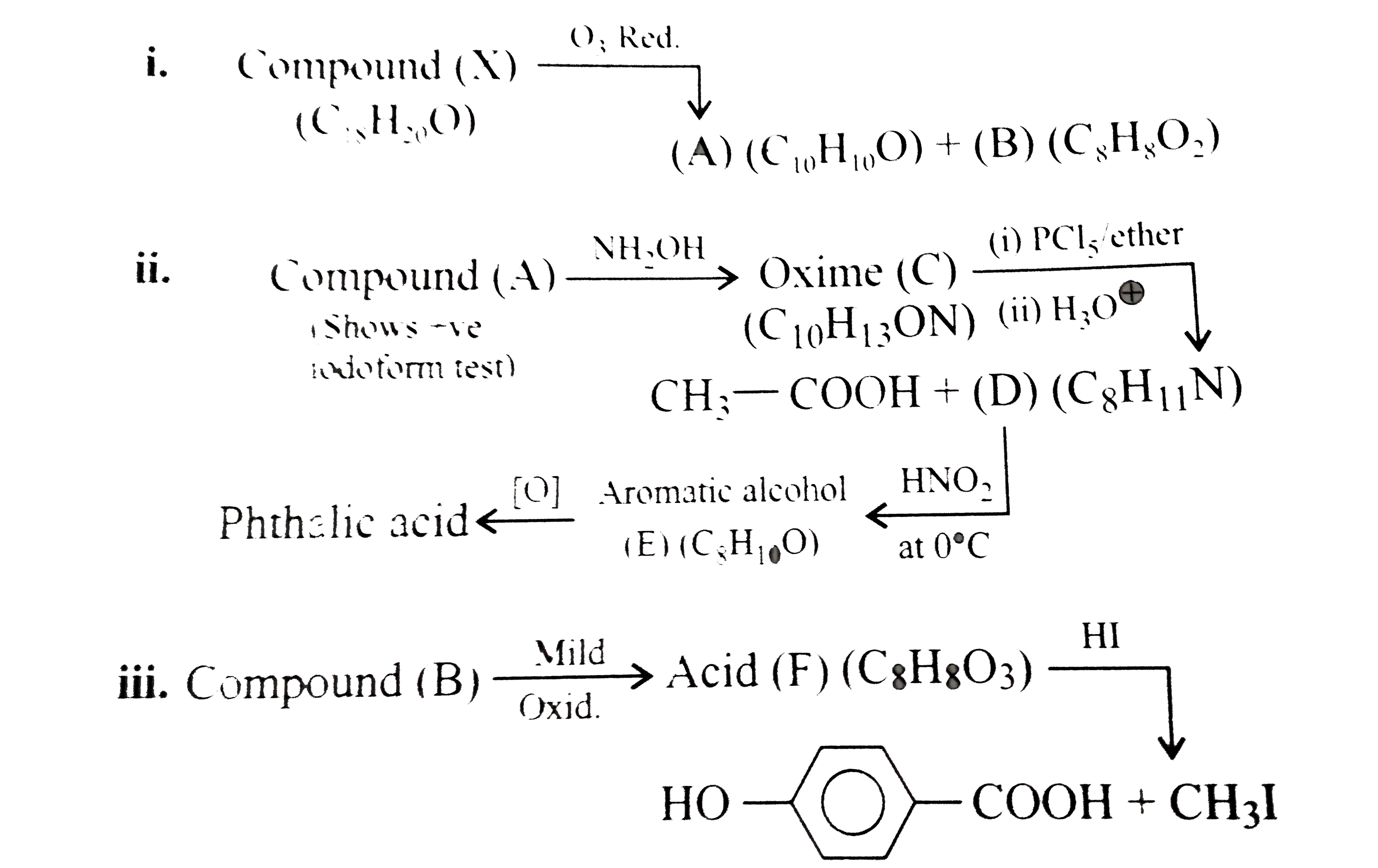
iv. Compound (X) has the highest melting point among its isomers.
Compound (X) is:A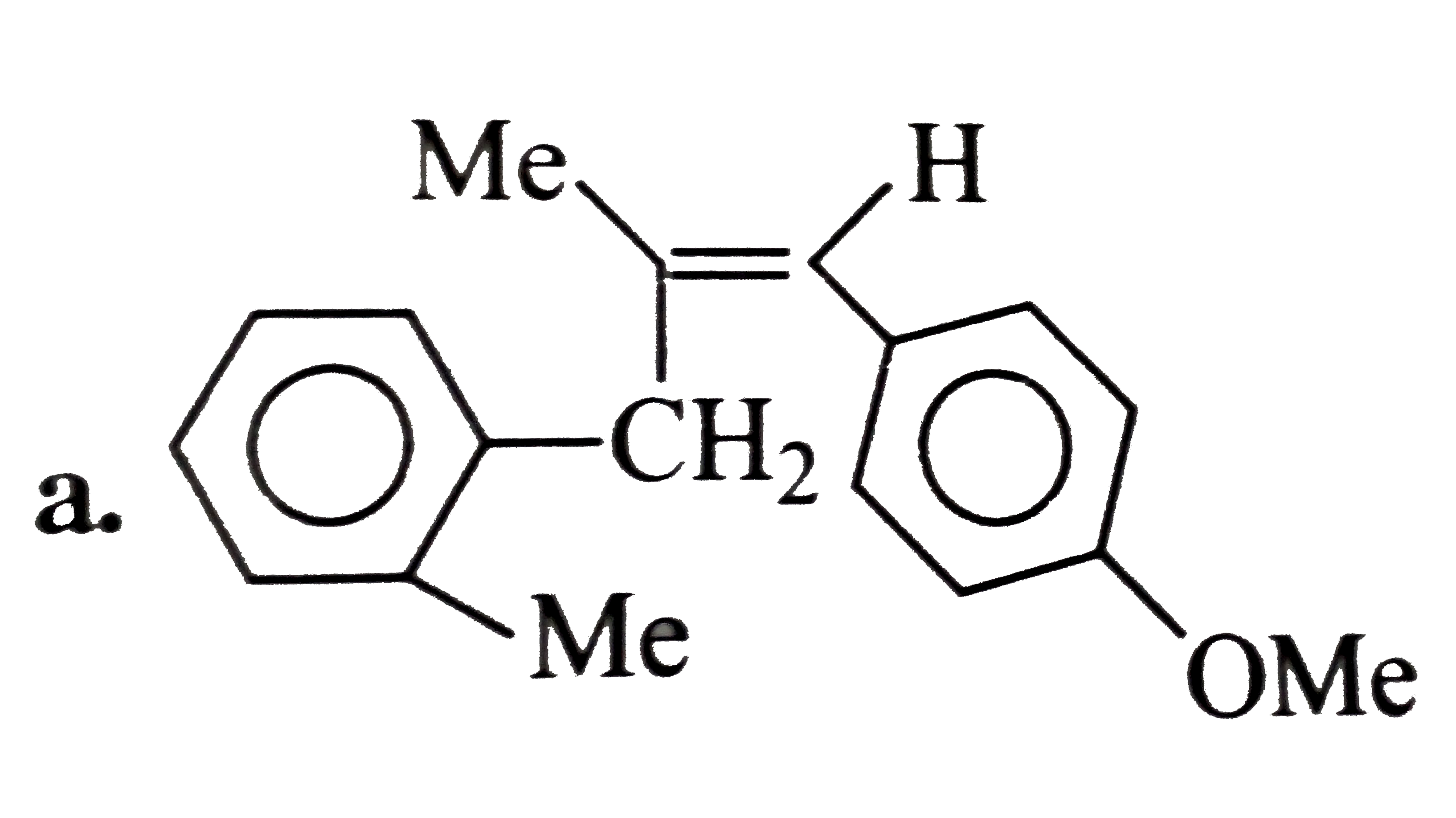 B
B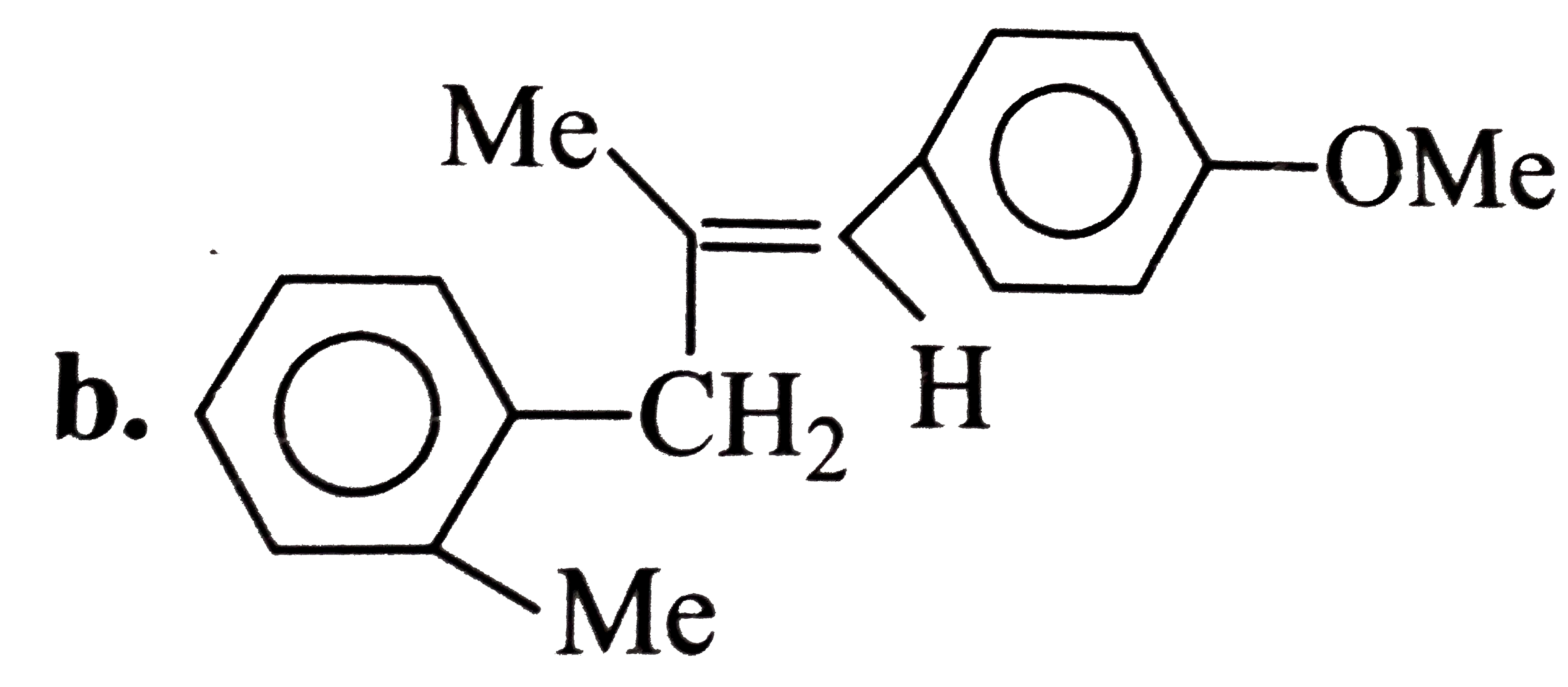 C
C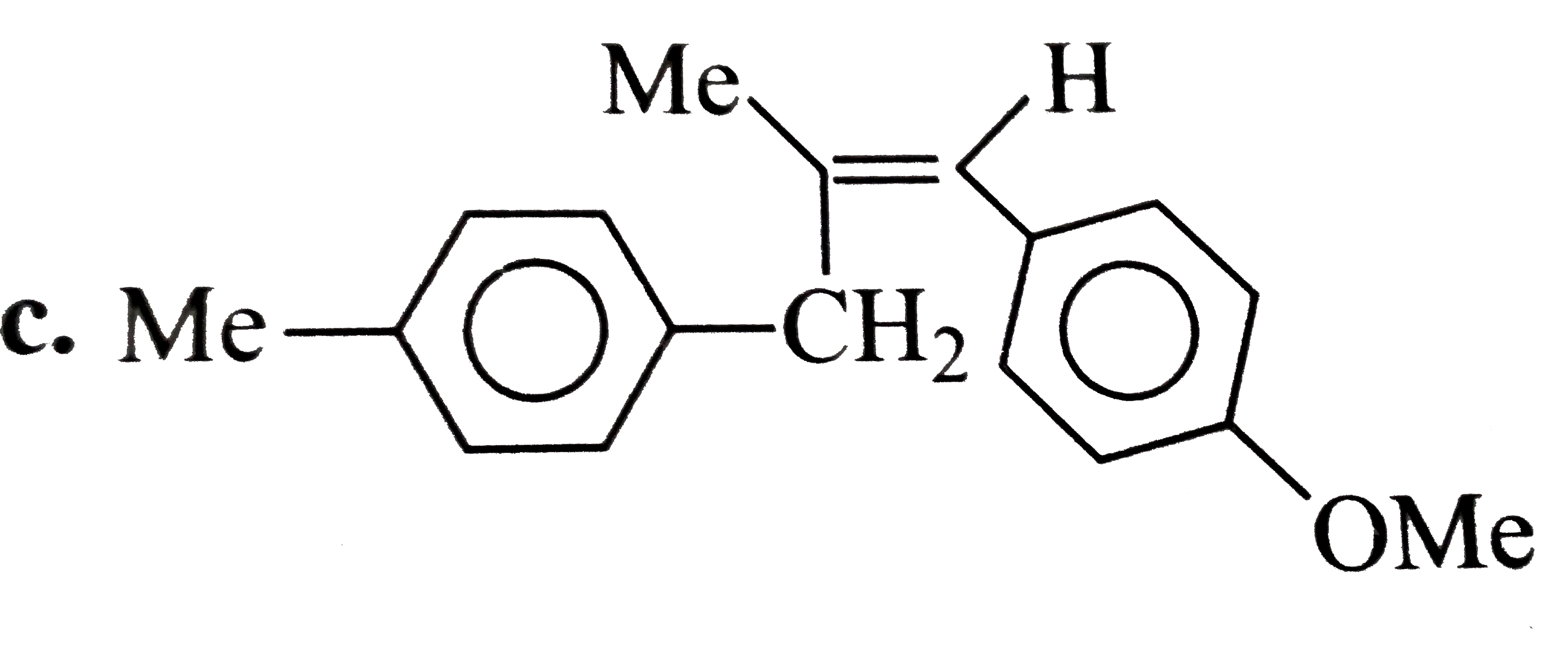 D
D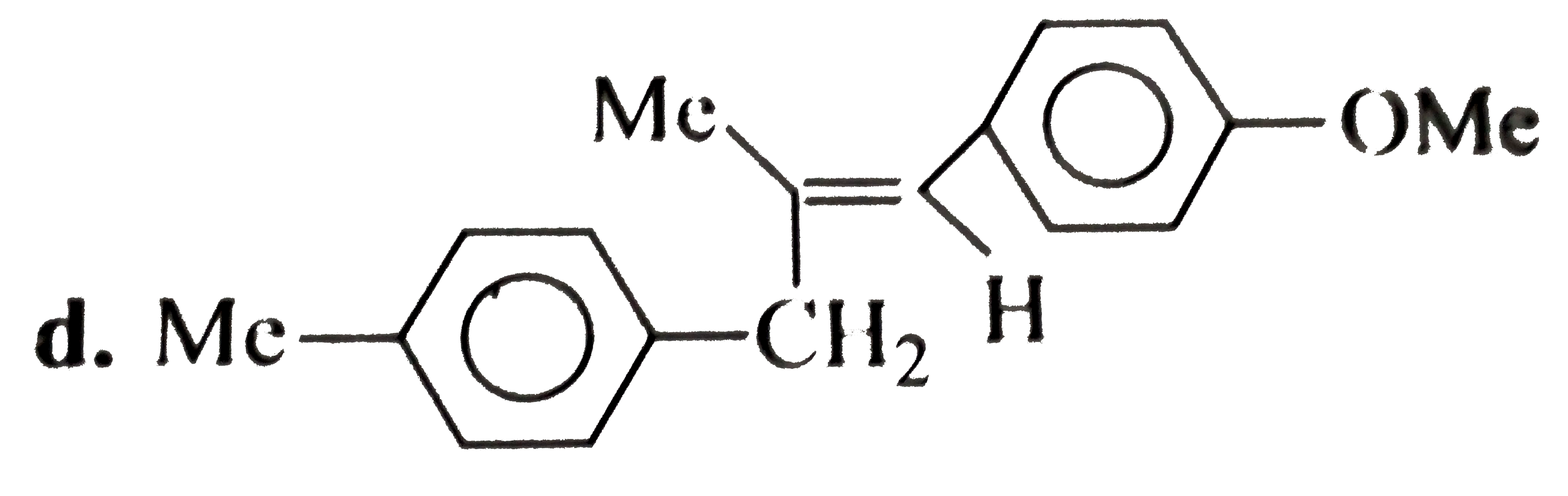
i.
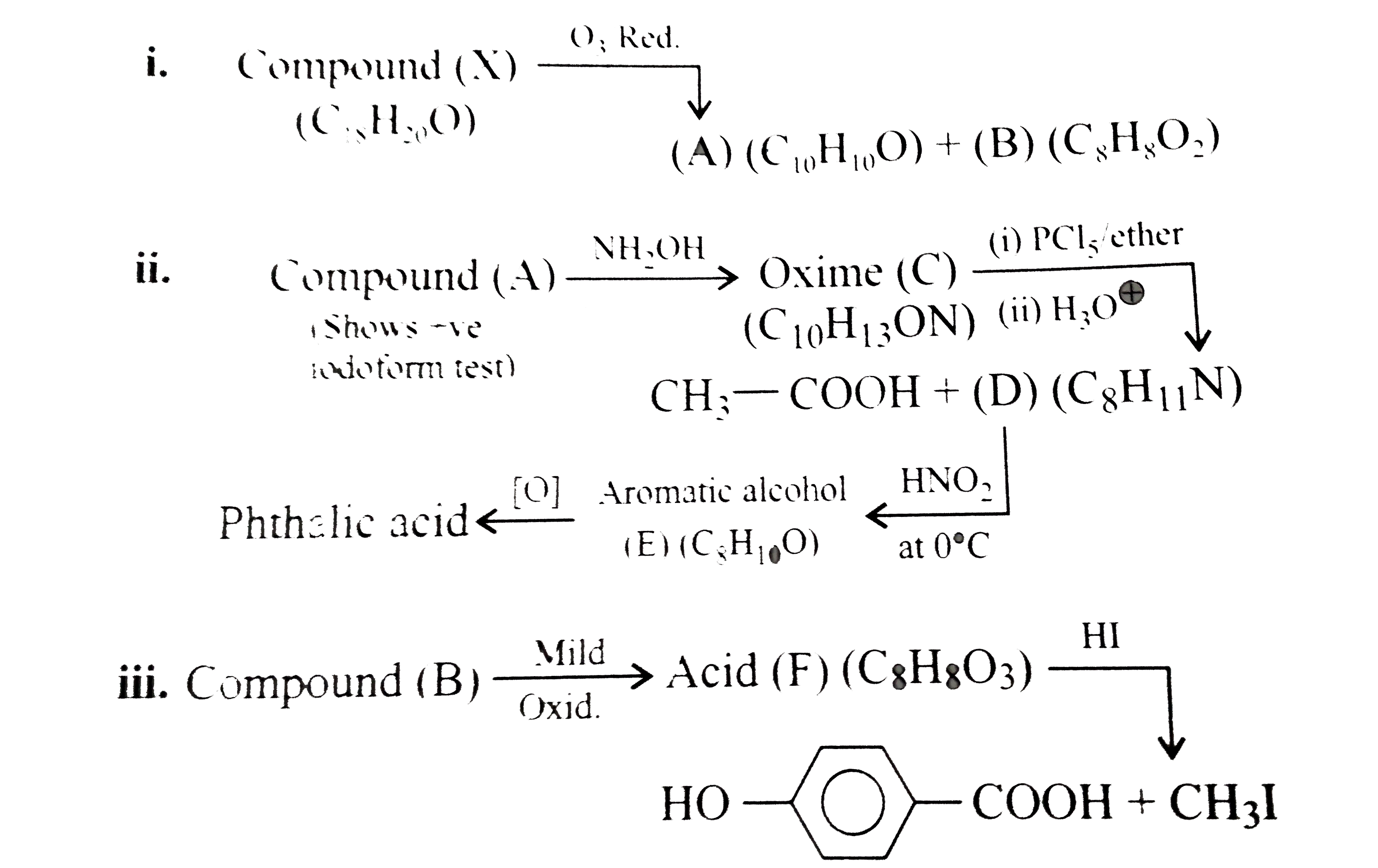
iv. Compound (X) has the highest melting point among its isomers.
Compound (D) is:A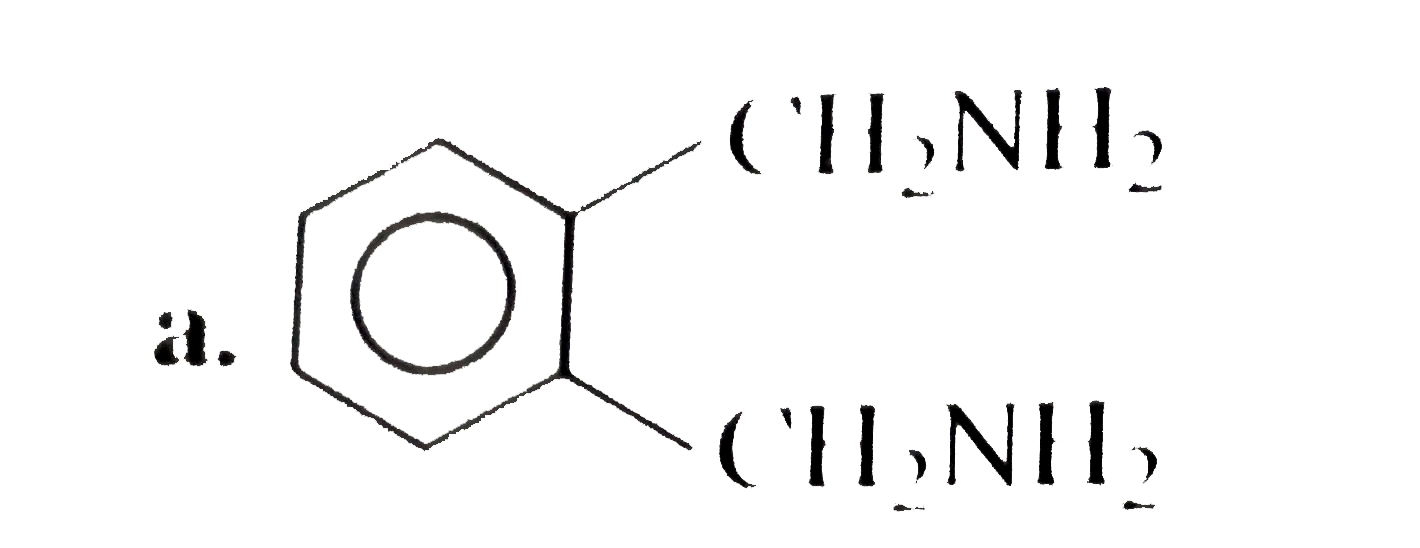 B
B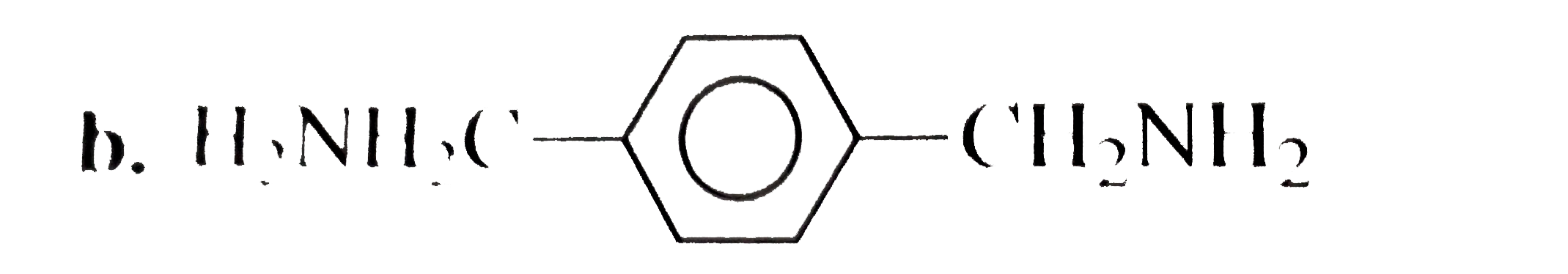 C
C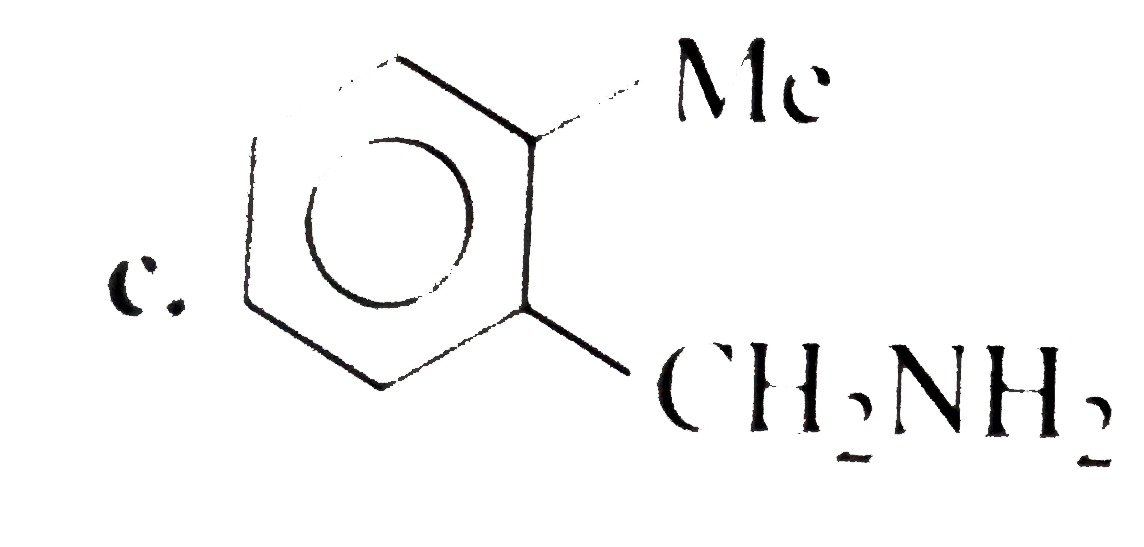 D
D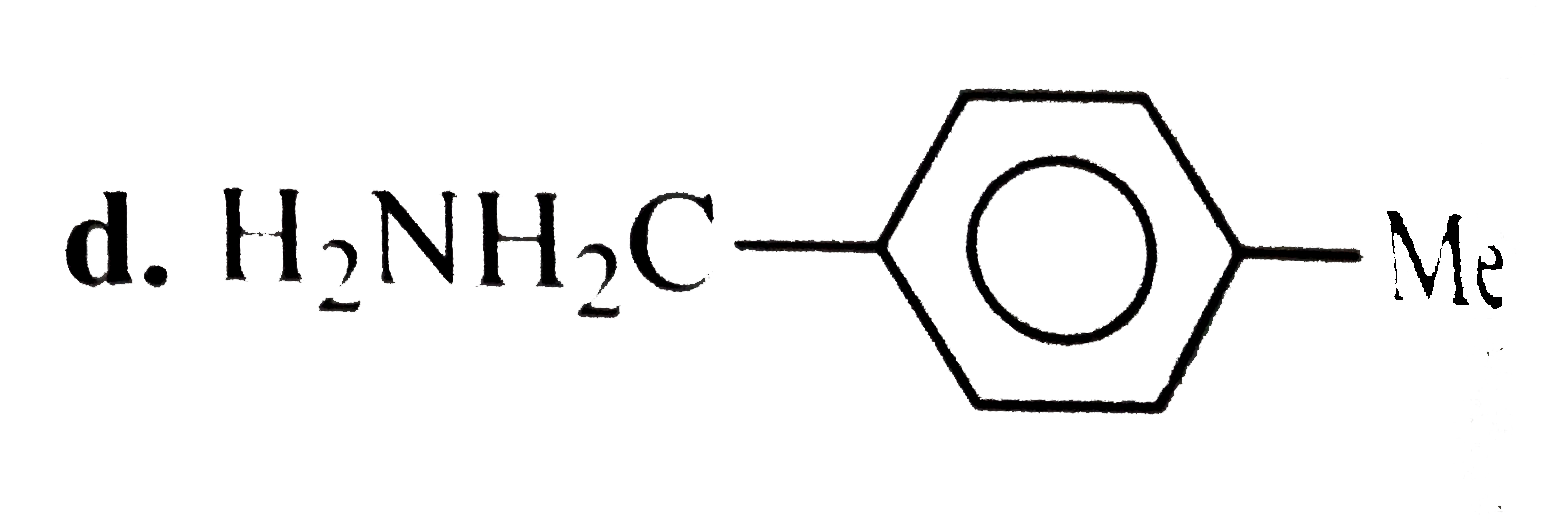
i.
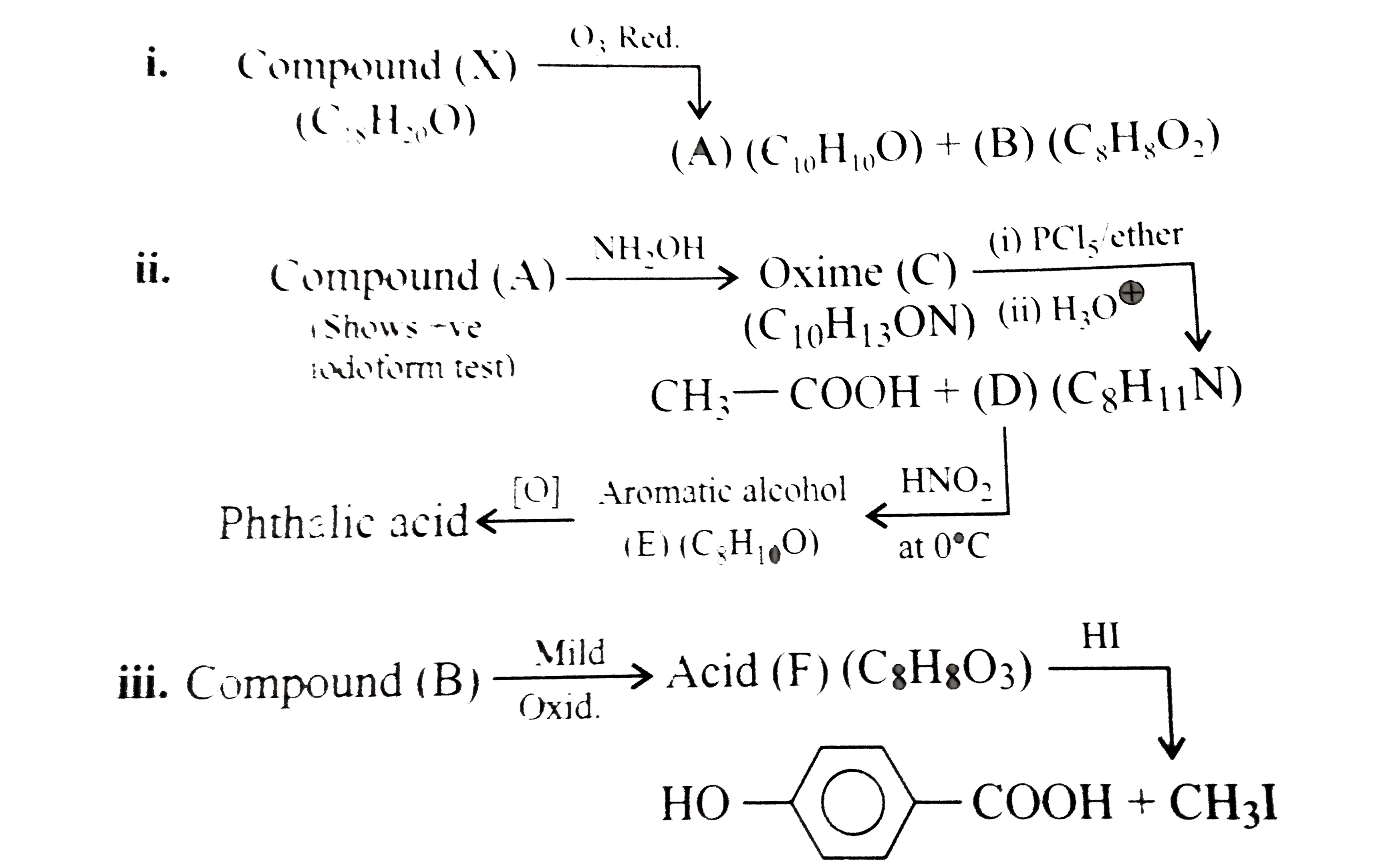
iv. Compound (X) has the highest melting point among its isomers.
Compound (C ) is:A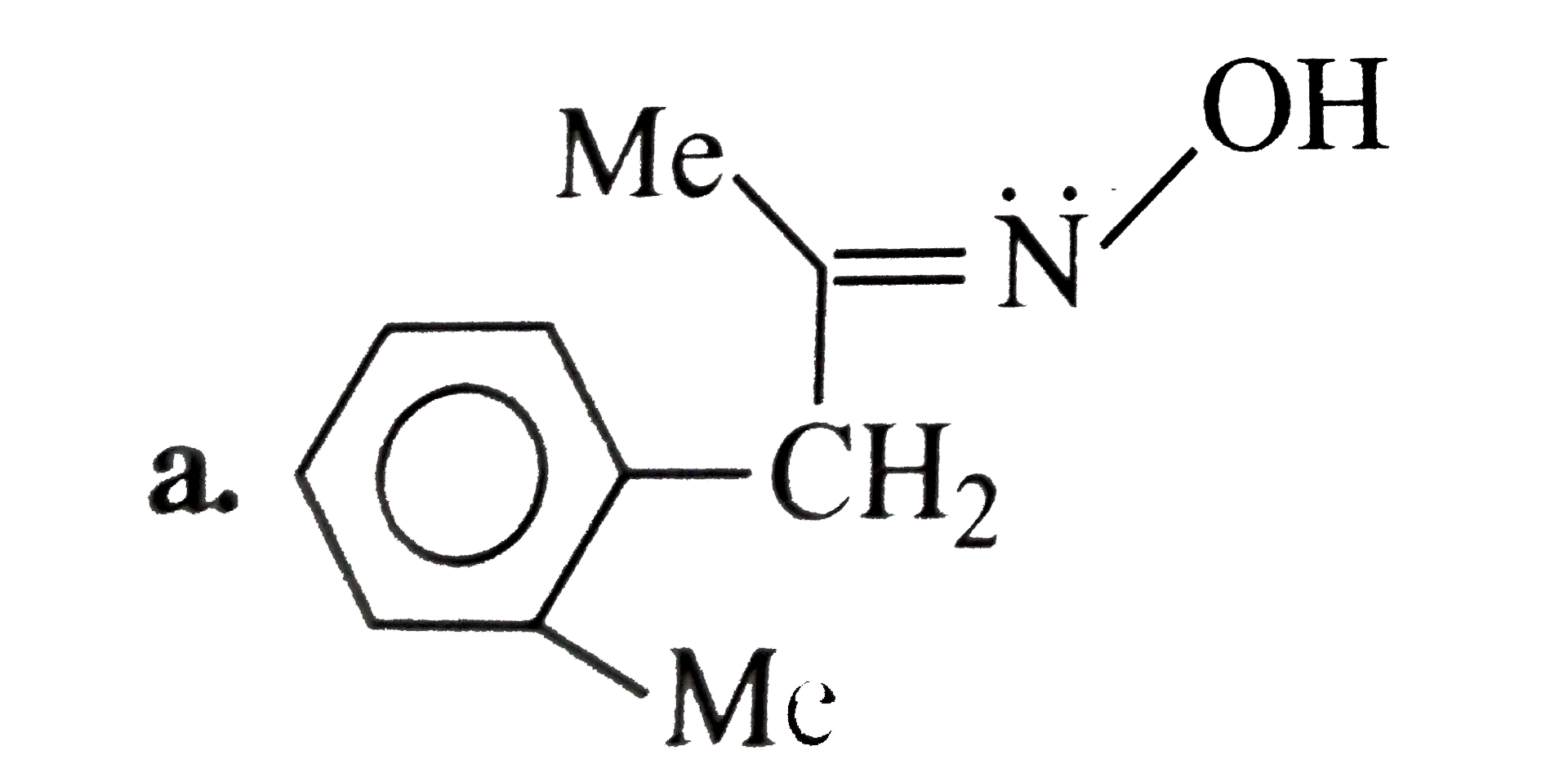 B
B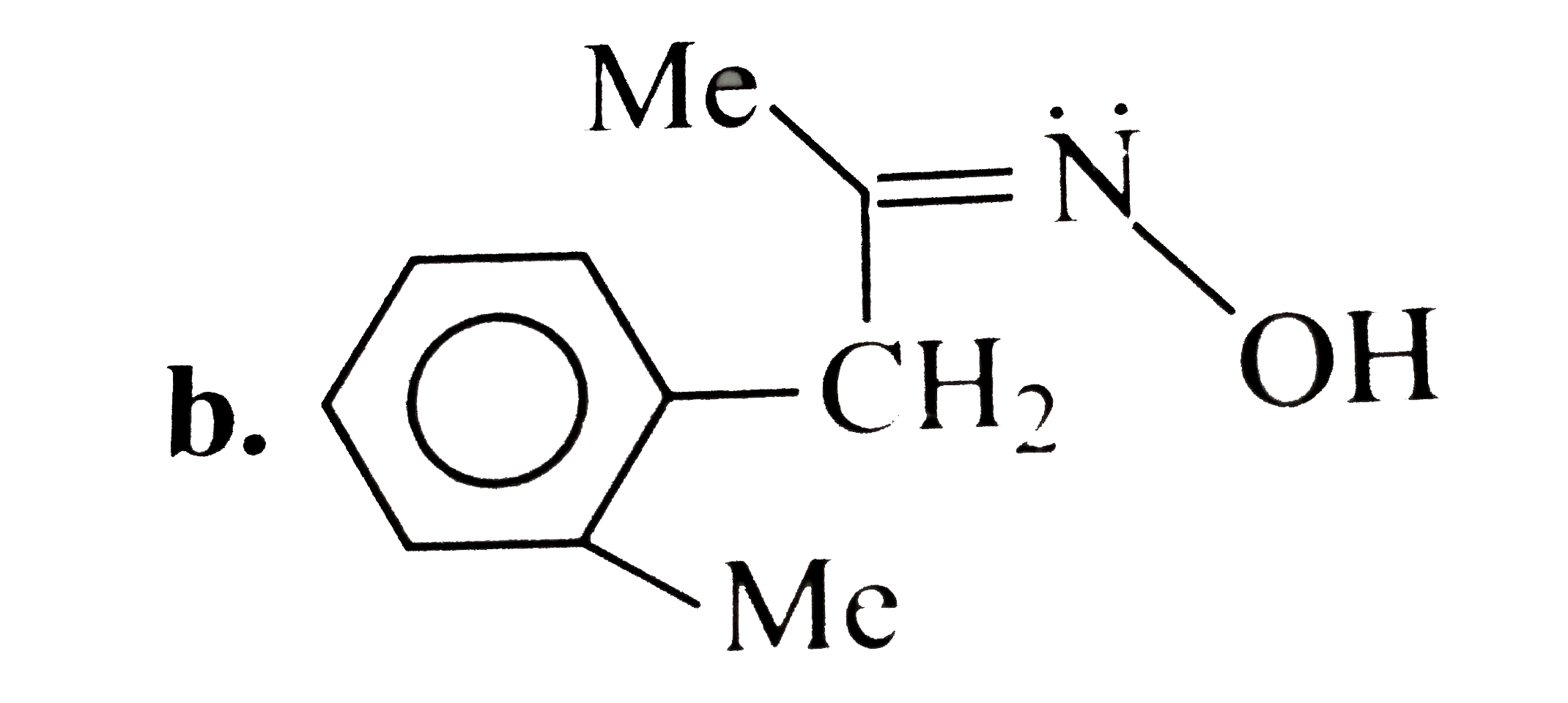 C
C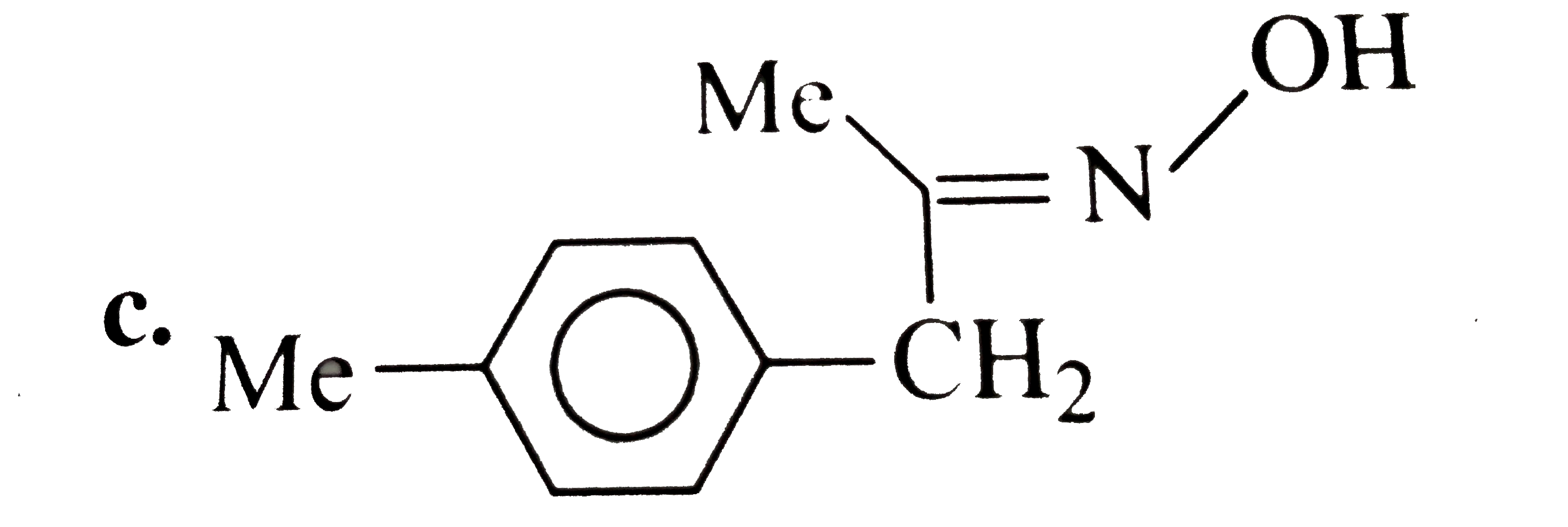 D
D