Carboxylic acid, although unreactive to alcohols, reacts in the presence of small amount of conc.H2SO4 or with 2−3% of HCl ?
RCOOH+R'OHH⊕⇐⇒RCOOR'+H2O
Which of the following statements is//are correct about the above reaction ?
(I) This reaction is called Fischer esterification reaction.
(II) The equilibrium is shifted to R.H.S if H2O is removed by azeotropic distillation with benzene.
(III) The reaction of RCOCl and R'OH to give ester (RCOOR') is irreversible and more feasible than esterification of RCOOH.
(IV) If the above esterification of RCOOH is carried out in excess of R'OH, the equilibrium is shifted to R.H.S.
A
(I),(II)
B
(I),(II),(III)
C
(I),(II),(IV)
D
All
Video Solution
Text Solution
The correct Answer is:D
All the statements are self-explanatory.
|
Similar Questions
Knowledge Check
Which of the following is correct for the equilibrium of the reaction ?
C(s)+H2O(g)⇔CO(g)+H2(g)ApH2∝pH2OBpH2∝√pH2OCpH2∝p2H2ODpH2∝p2H2OpCOWhich of the following statements are correct about the following reaction :
R−O∣∣C−OH+R'OHH⊕⇐⇒RCOOR'+H2OARate =K[RCOOH][R'OH][H+]. First order in acid, alcohol, and H⊕ , third order overall.BRate : K[RCOOH][R'OH]. First order in acid and alcohol , second order overall.CSlow and rate-determining step (R.D.S) is the addition of alcohol to the protonated acid.DSlow and rate-determining step (R.D.S) is the addition of acid to the protonated alcohol.EtCOCl(A)(i)H2N.OH−−−−−−−→(ii)ӨOH(B)EtOH−−−→(C)
Which of the following statements are correct about the given reaction ?AThe compouds (B) and (C), respectively, are Et−C≡N and EtNH2.BThe compounds (B) and (C), respectively, are EtN=C=O and EtNHCOOEt.CThe reaction is known as Lossen rearrangement reaction.DThe reaction proceeds via the formation of acyl carbene (EtCO..CH) as the intermediate species.
Which of the following statements is wrong about the transesterification reaction, catalysed by H3O⊕(H2SO4ordryHCl) or ROӨ(EtONa) ?
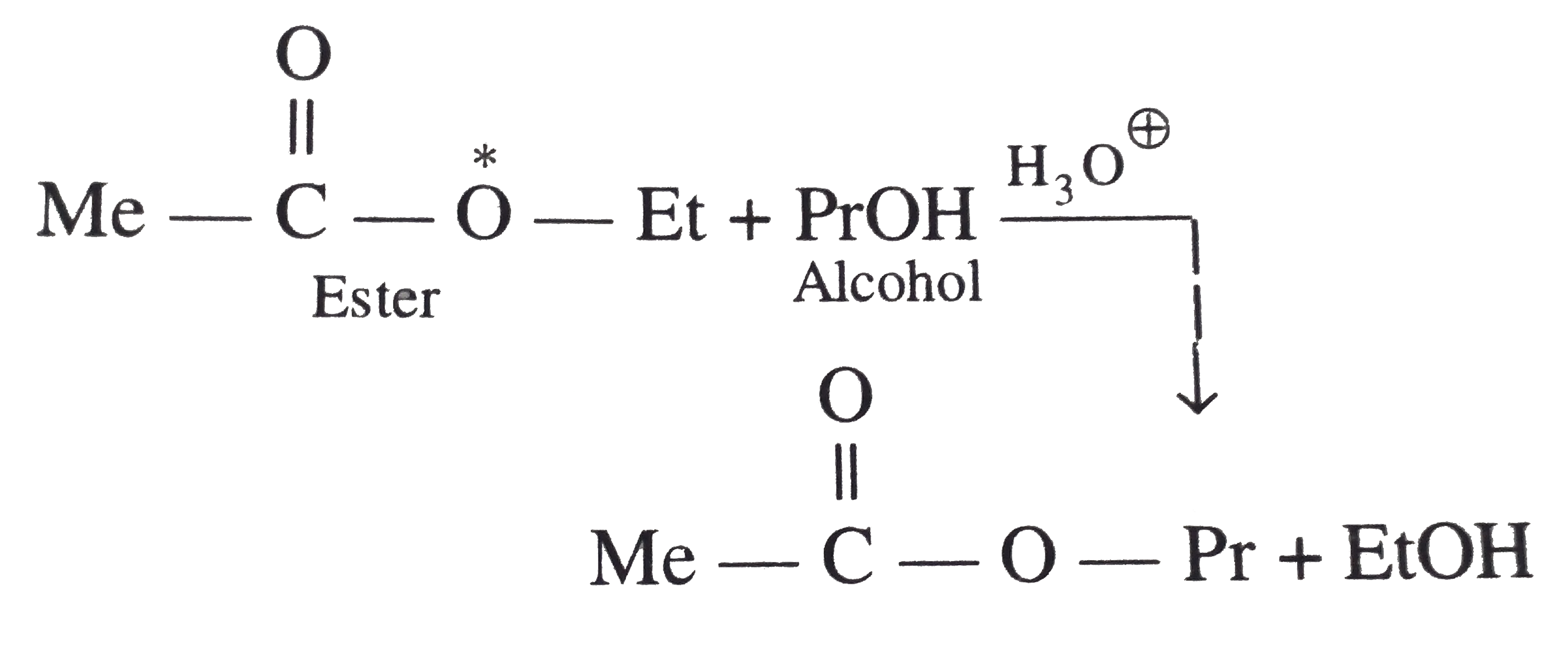 .
.
View SolutionIn esterification reaction , conc. H2SO4, acts as
View SolutionC12H22O11Conc.−−−→H2SO412C+11H2O
Which of the following obtained in the above reaction?
View SolutionIn esterification reaction, the role of conc. H2SO4 is
View SolutionR−OH−HX→RX+H2O
In the above reaction, the reactivity of alcohols is
View Solution
