(a). Glucose and fructose form the same osazone, what does it prove?
(b). Why more than 3molofPhNHNH2 do not react with glucose and fructose and how does PhNHNH2, which is a powerful reducting agent, act as an oxidising agent in this case?
(a)
Glucose and furctose form the some osazoe which proves that the configuration of OH group or stereochemistry at C−3,C−4,C−5, and C−6 in glucose and fructose is same.
(b). Mechanism: (Amadori rearrangement)

In the above mechanism, we find that PhNHNH2 does not act as an oxidising agent.
The reaction takes place only up to two C atoms because the intermediate osazone formed can be stabilised by H−bonding between N and H by forming a six-membered ring as shown below.
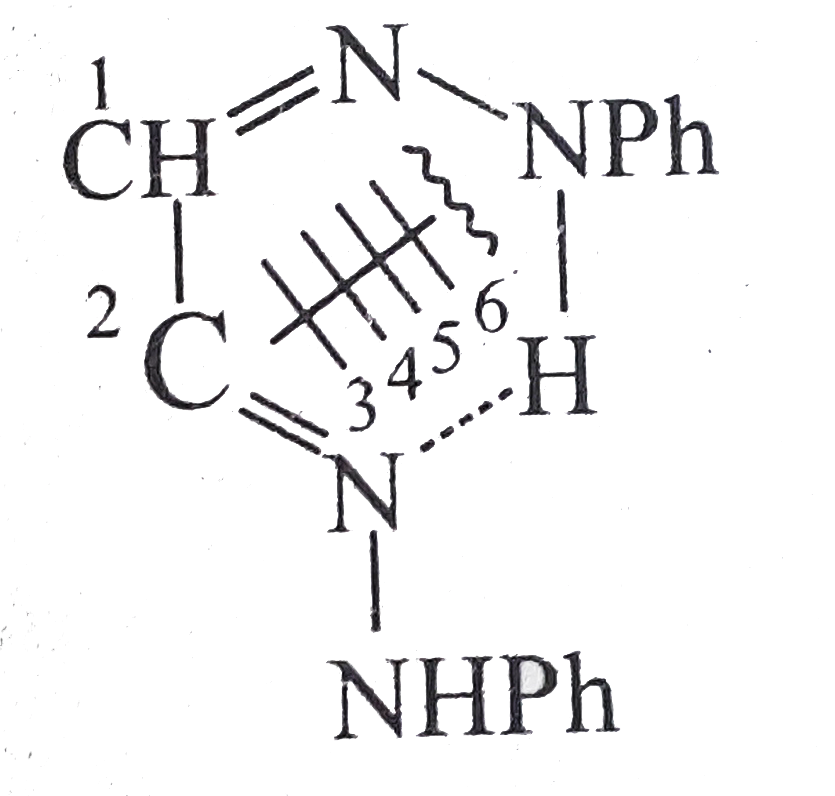
Similar Questions
Knowledge Check
In which of the following reaction does SO2 acts as oxidising agent?
Aacidified KMnO4Bacidified K2Cr2O7Cacidified C2H5OHDH2S
Why does O3 act as a powerful oxidising agent?
View Solution(a) How does H2O behave as a bleaching agent?
(b) What causes temporary and permanent hardness of water?
View SolutionD-glucose and D-fructose have different structures. Why do they form the same product on reacting with excess of phenyl hydrazine ?
View Solution(a) What is the covalence of nitrogen in N2O5?
(b) BiH3 is stronger reducing agent than SbH3, why ?
View Solution(a) Account for the following observations :
(i) SF1 is easily hydrolysed whereas SF6 is not easily hydrolysed.
(ii) Chlorine water is a powerful bleaching agent.
(iii) Bi (V) is a stronger oxidising agent than Sb(V)
(b) What happens when :
(i) White phosphorus is heated with concentrated NaOH solution in an inert atmosphere of CO2.
(ii) XeF6 undergoes partial hydrolysis.
(Give the chemical equations involved).
View SolutionGlucose + Fructose + Monnose PhNHNH2−−−−−−→(Excess) How many osazone ?
View SolutionMeso-2,3-dibromobutane reacts with iodide ion more rapidly than does (±) compounds. What are the respective alkenes formed ? Explain.
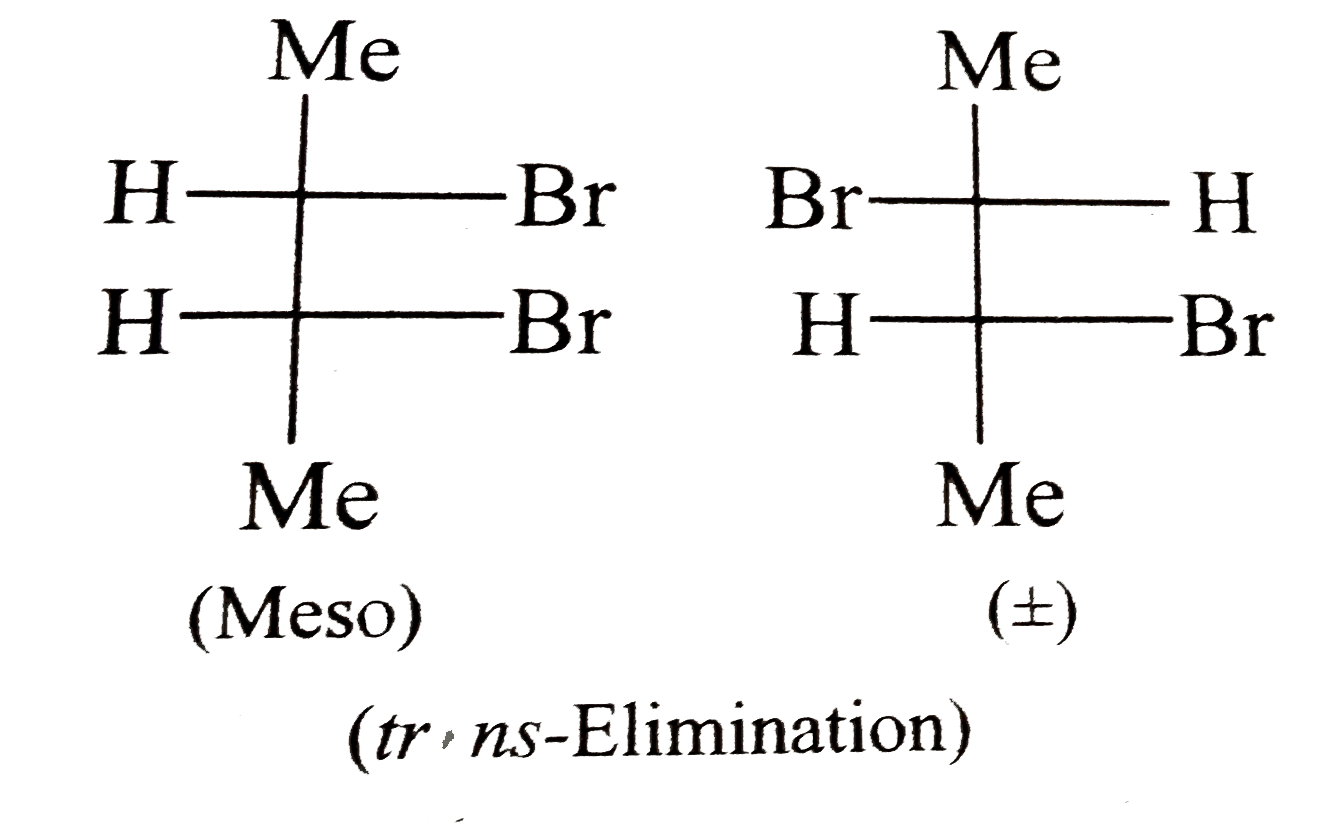 .
.
View Solution

