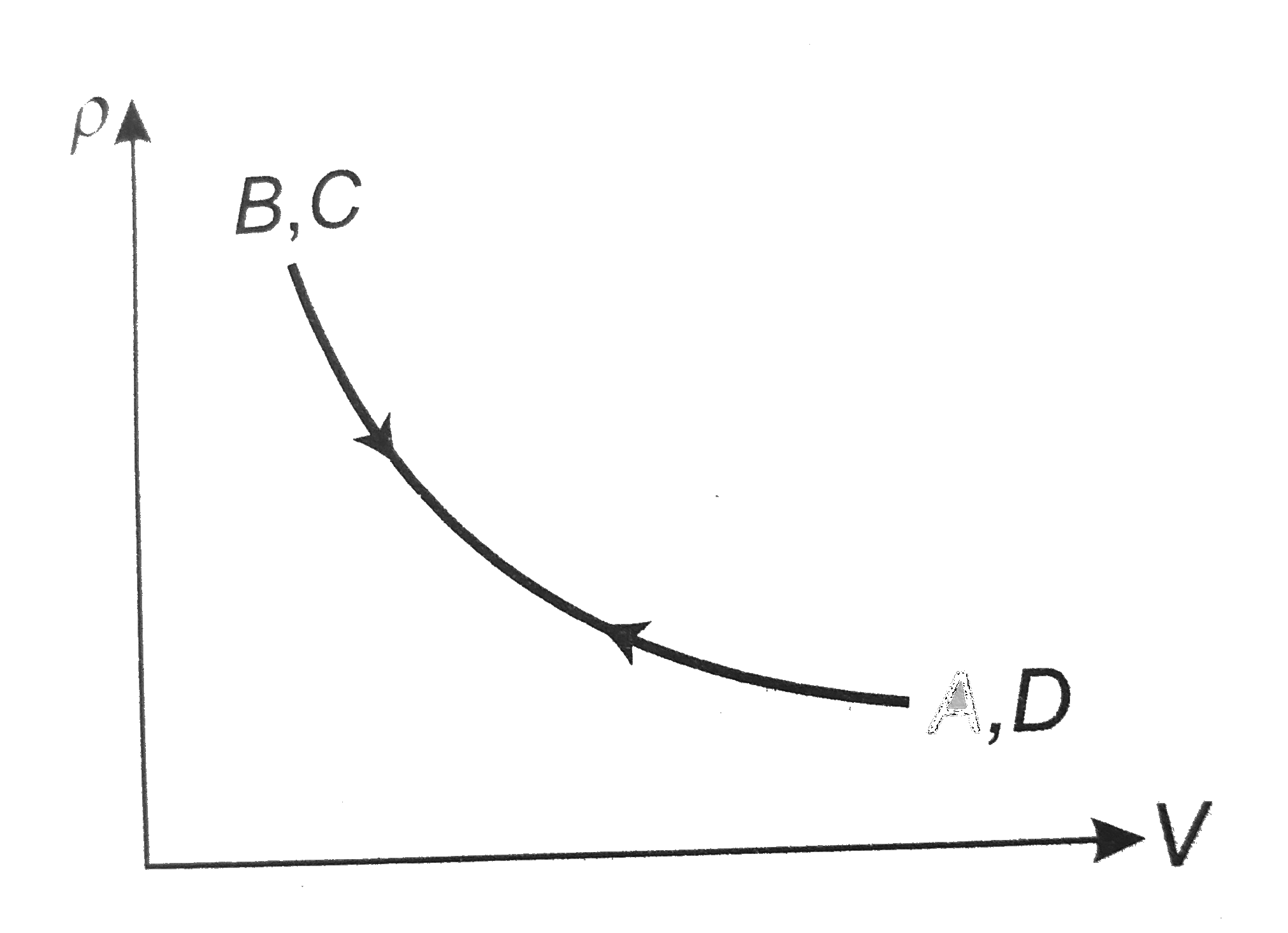
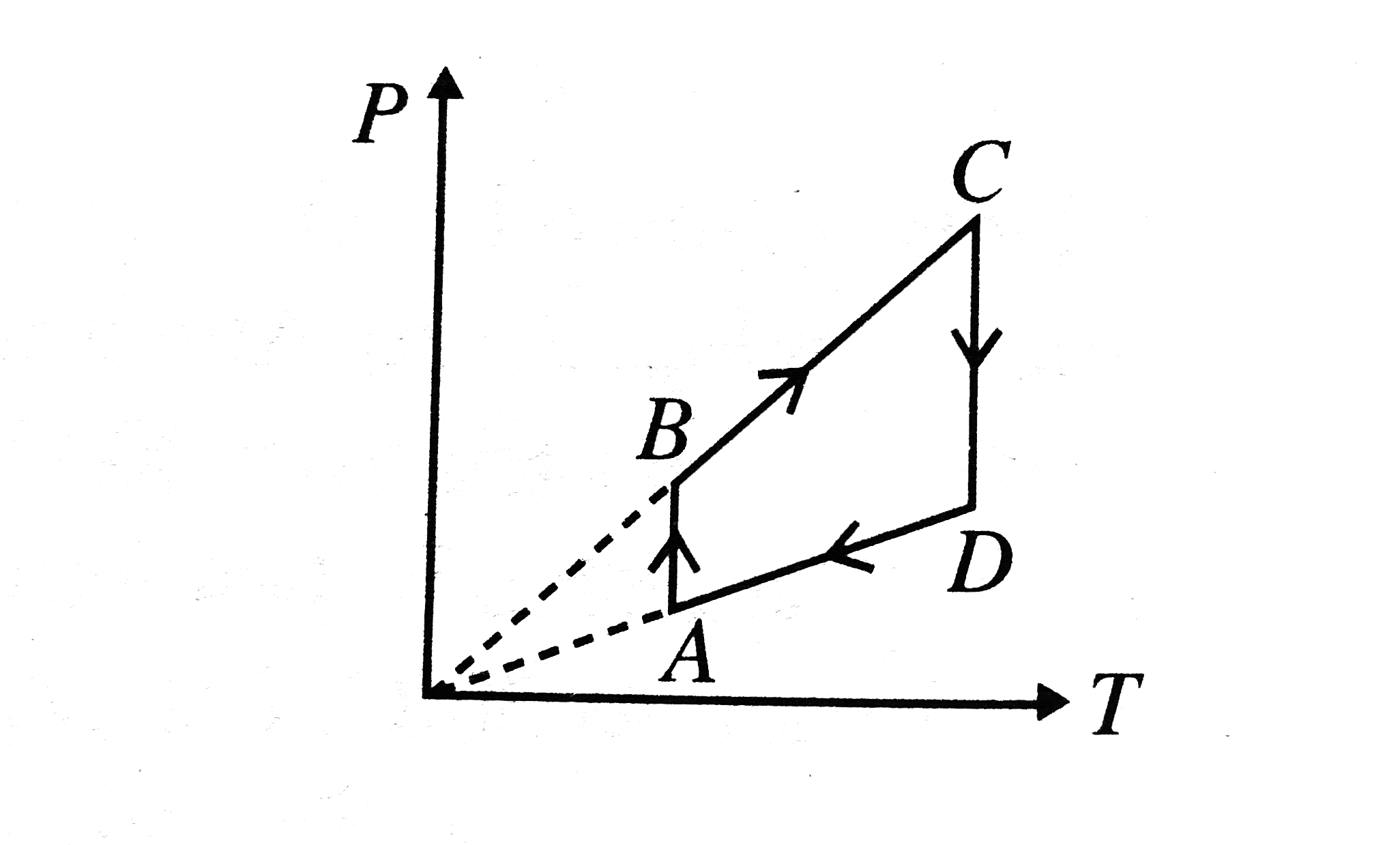
Density vs volume graph is shown in the figure. Find corresponding pressure vs temperature graph
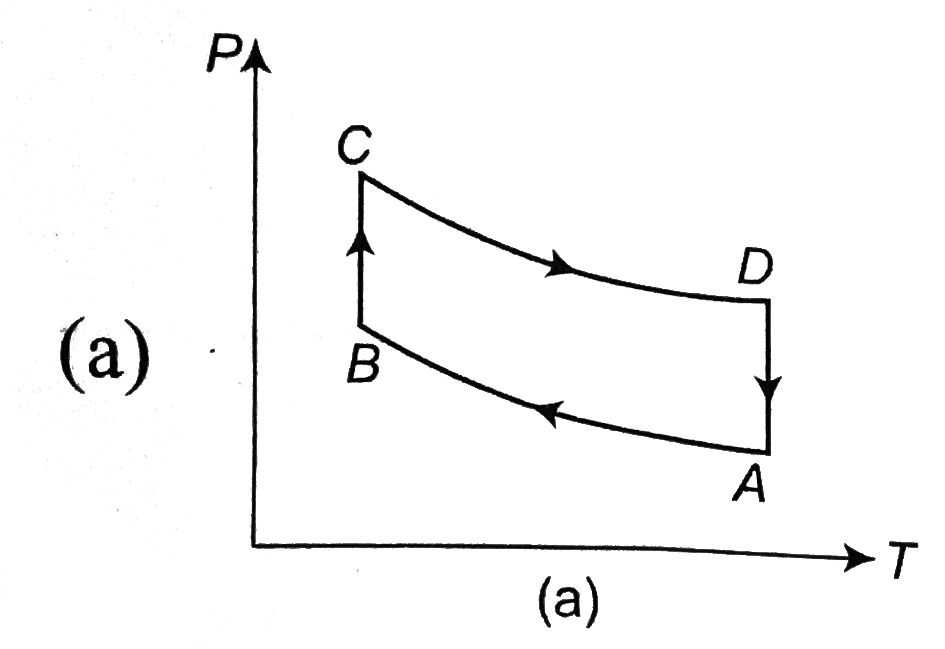
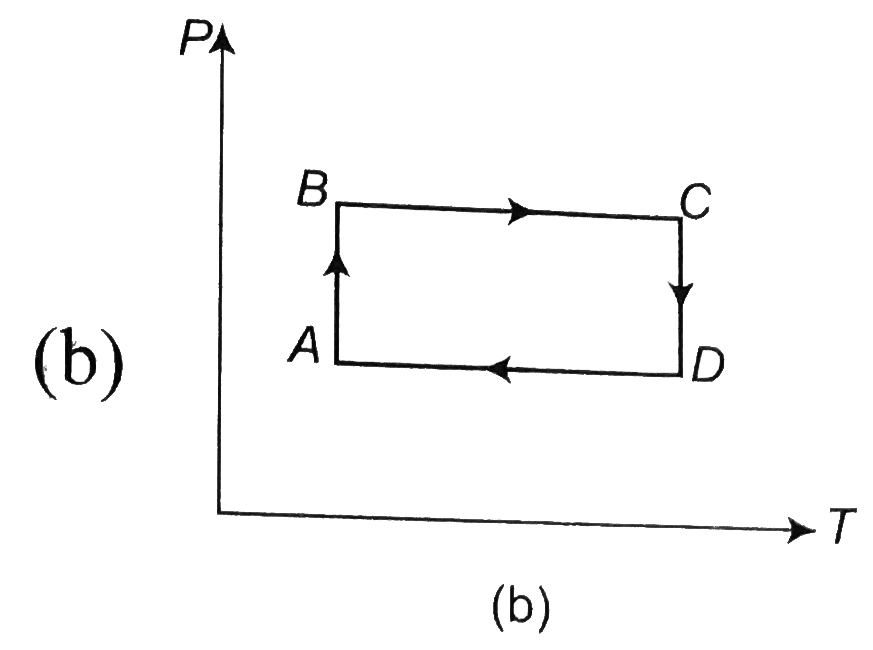
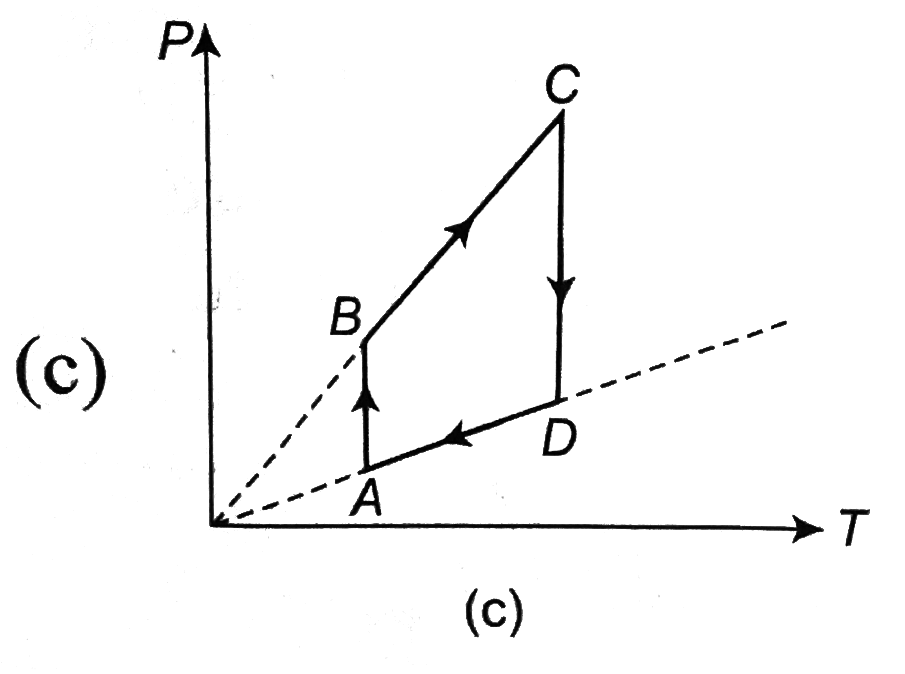
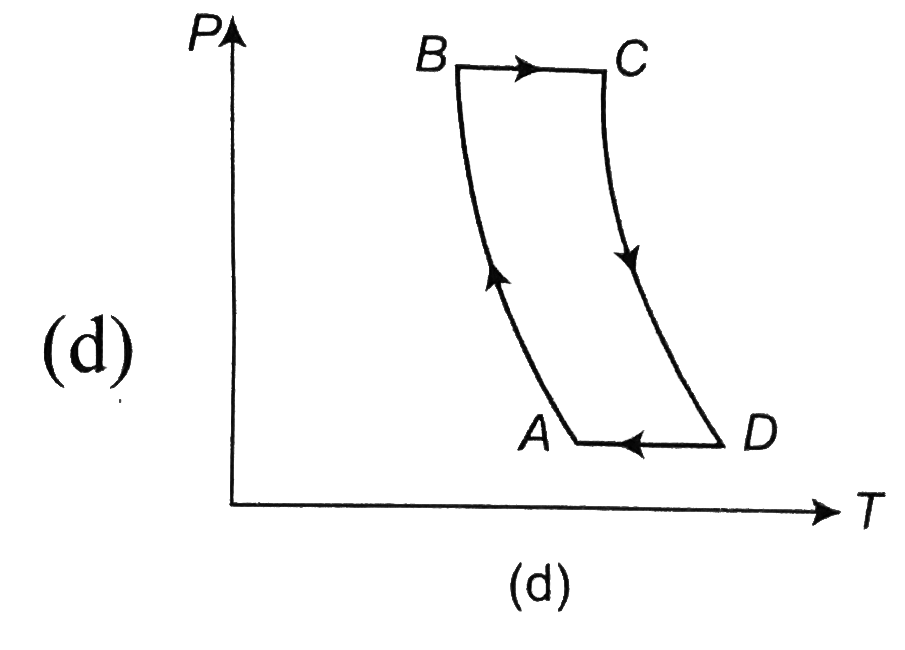
The correct Answer is:C
press∝1V in the preocess AB.
(V decrease, ρ increases)
In isothermal process (T = constant), ρ∝1V
As V decreases, P increases.
so, the ρ increase, V decrease (ρV curve) and P increase for V decrease (P−T curve)
Options (b) and (c) may be correct.
But process B−C is shown by a dot in (ρ−V) curve.
It means BC is a isochoric process.
Process BC in option (c) is a isochoric process because P∝T.
Similar Questions
Pressure temperature (p-1) graph of n moles of an ideal gas is shown in the figure. Among the following four graphs, which are the corresponding pressure volume (p-V) and density pressure (p-p) graphs?
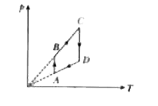
View SolutionThe graph shown in figure is exponential. Write down the equation corresponding to the graph.
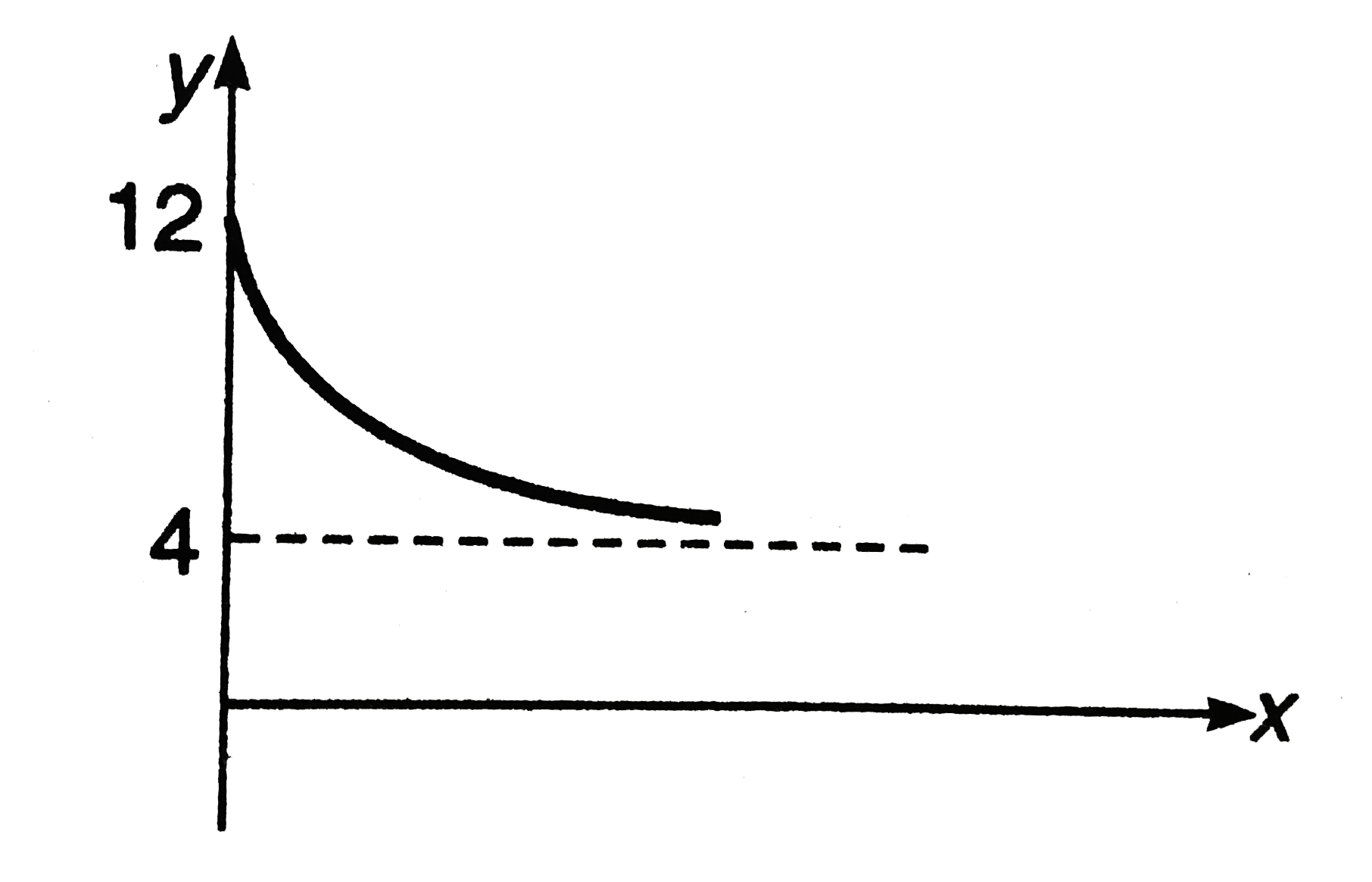
View Solution
Knowledge Check
Pressure versus temperature graph of an ideal gas at constant volume V is shown by the straight line A. Now mass of the has is doubled and the volume is halved, then the corresponding pressure versus temperature graph will be shown by the line
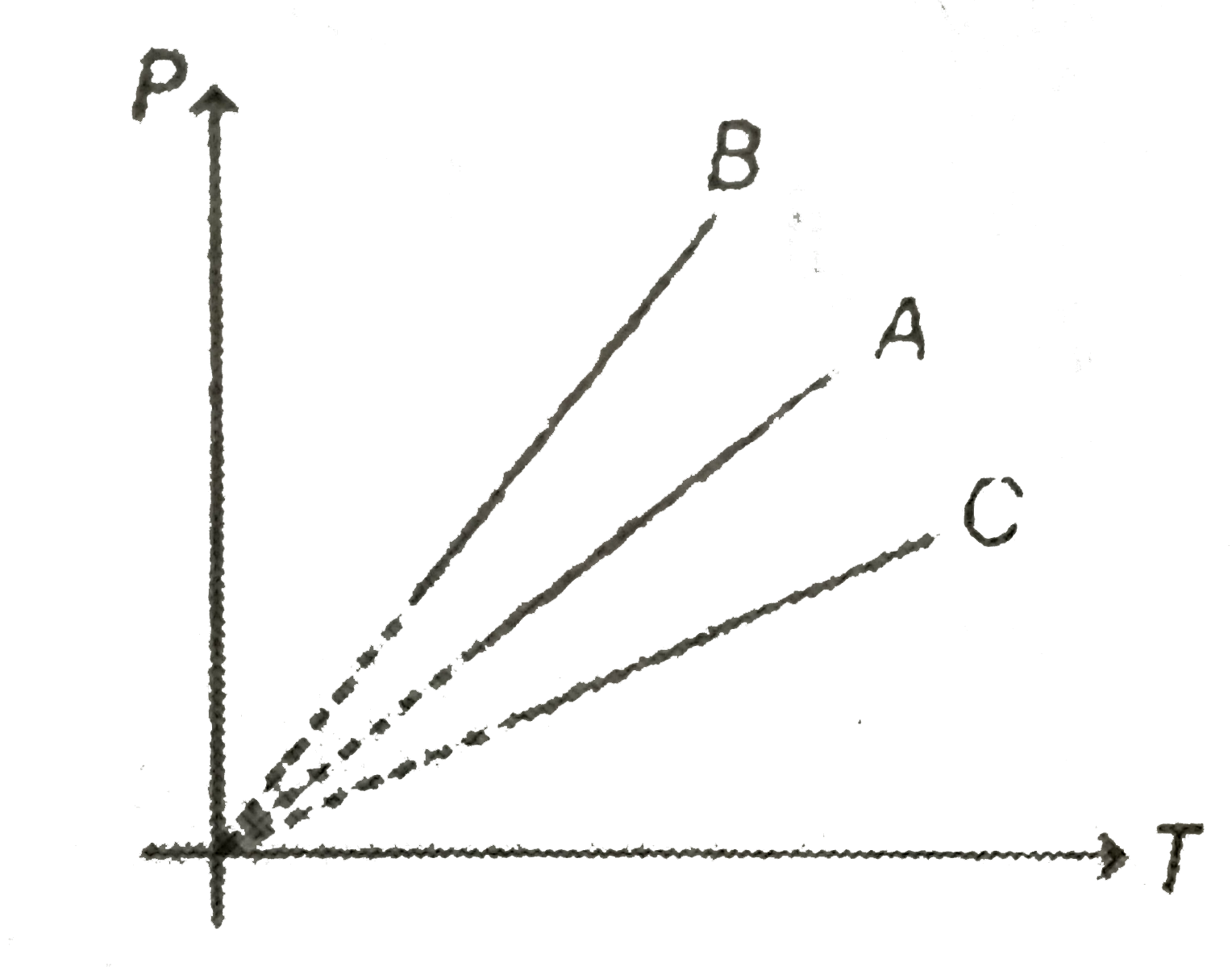 AABBCCDNone of these
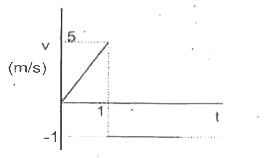
AABBCCDNone of theseThe velocity time graph for a particle moving along X-axis is shown in the figure. The corresponding displacement time graph is correctly shown by
 A
A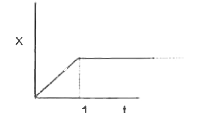 B
B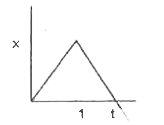 C
C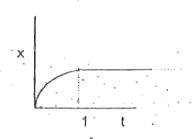 D
D
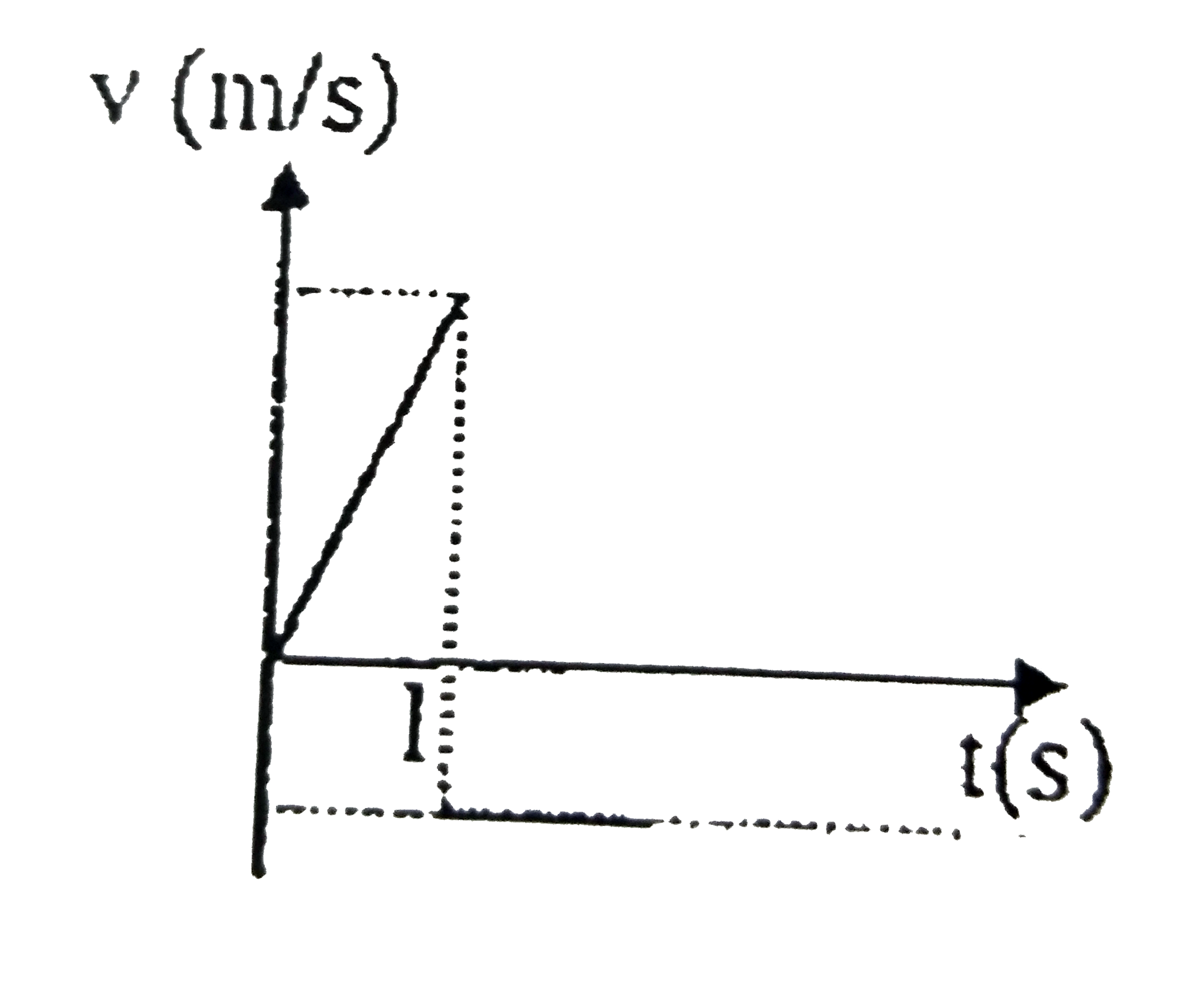
The velocity time graph for a particle moving along x-axis is shown in the figure. The corresponding displacement time graph is correctly shown by
 A
A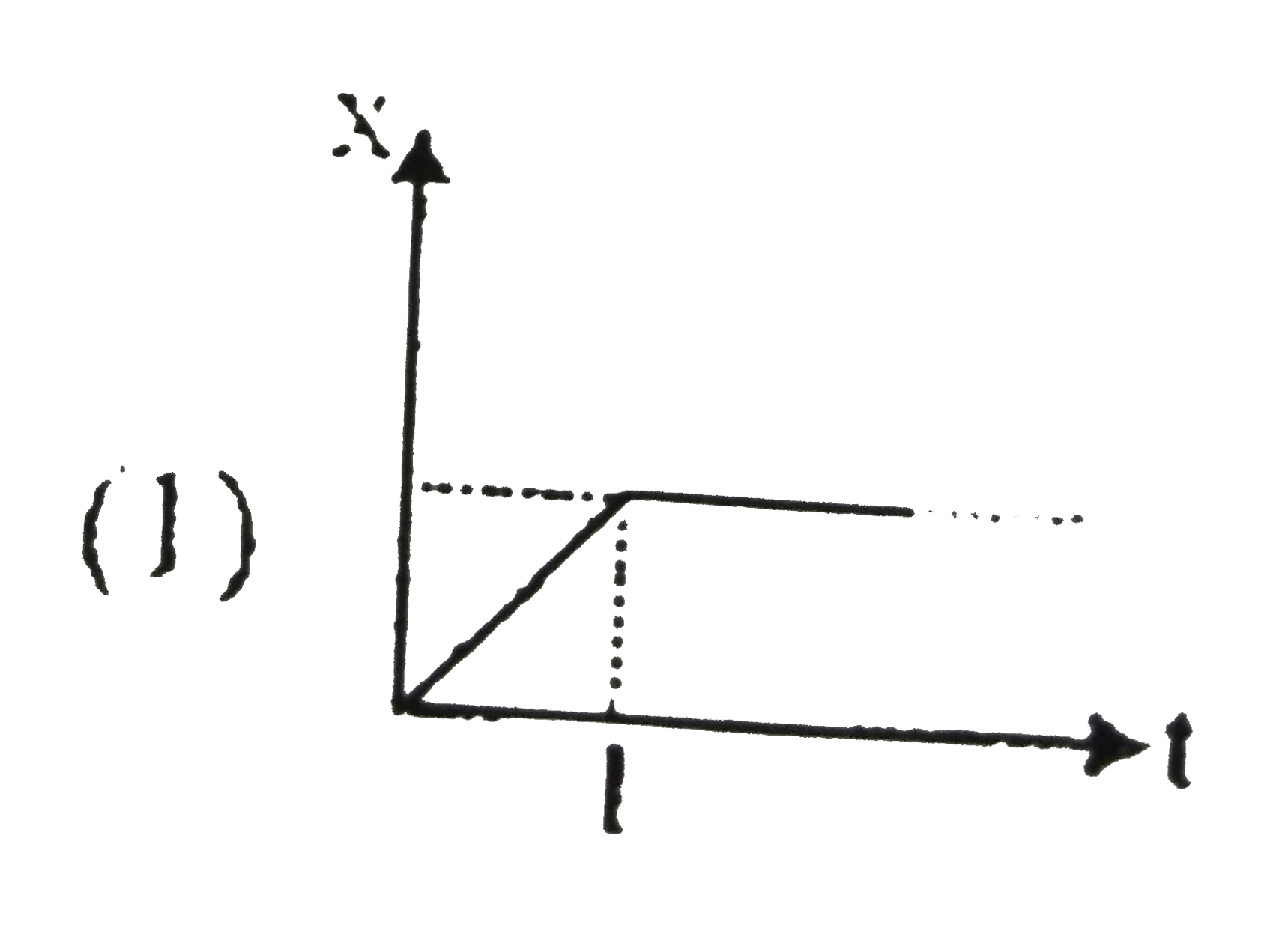 B
B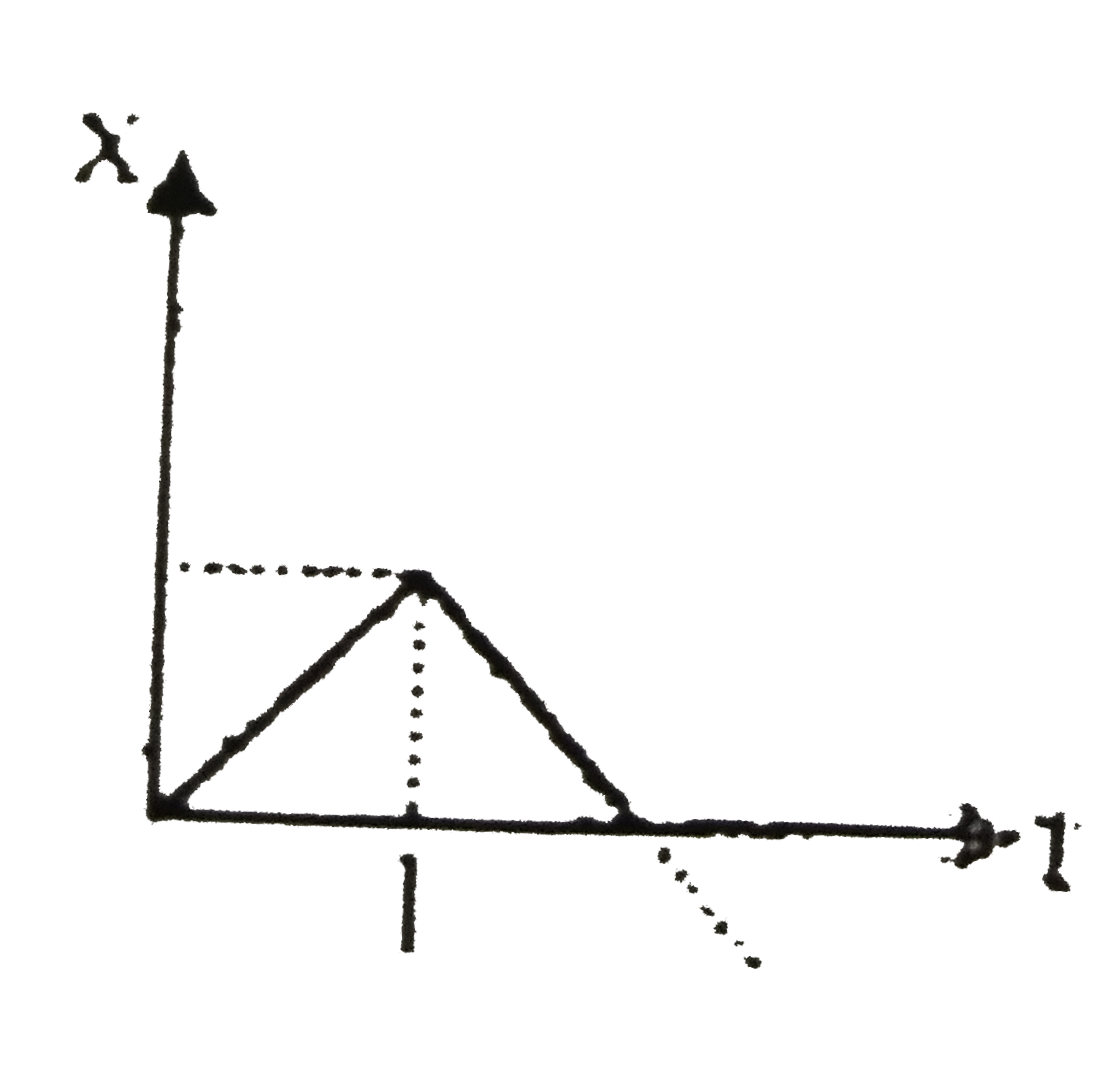 C
C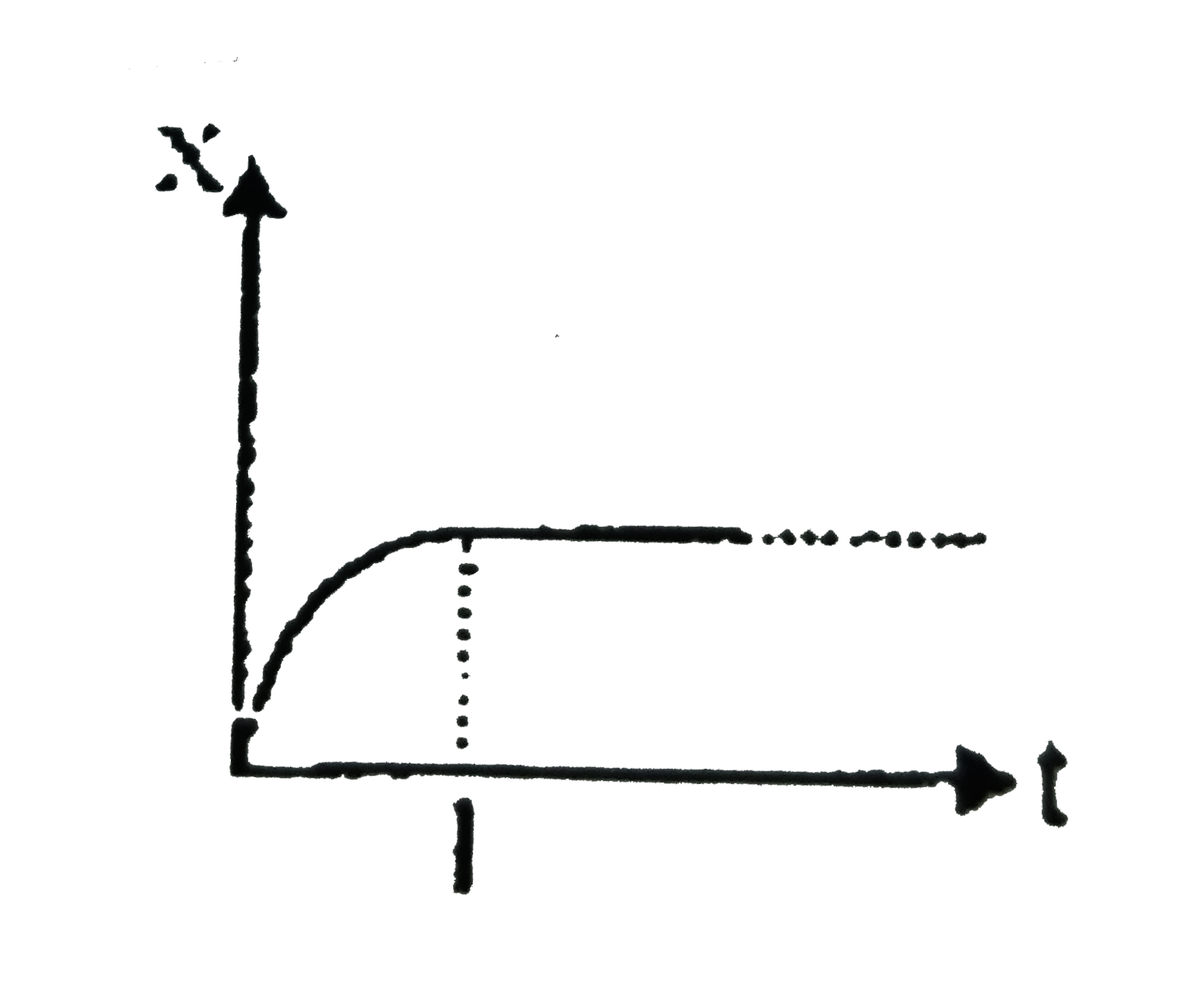 D
D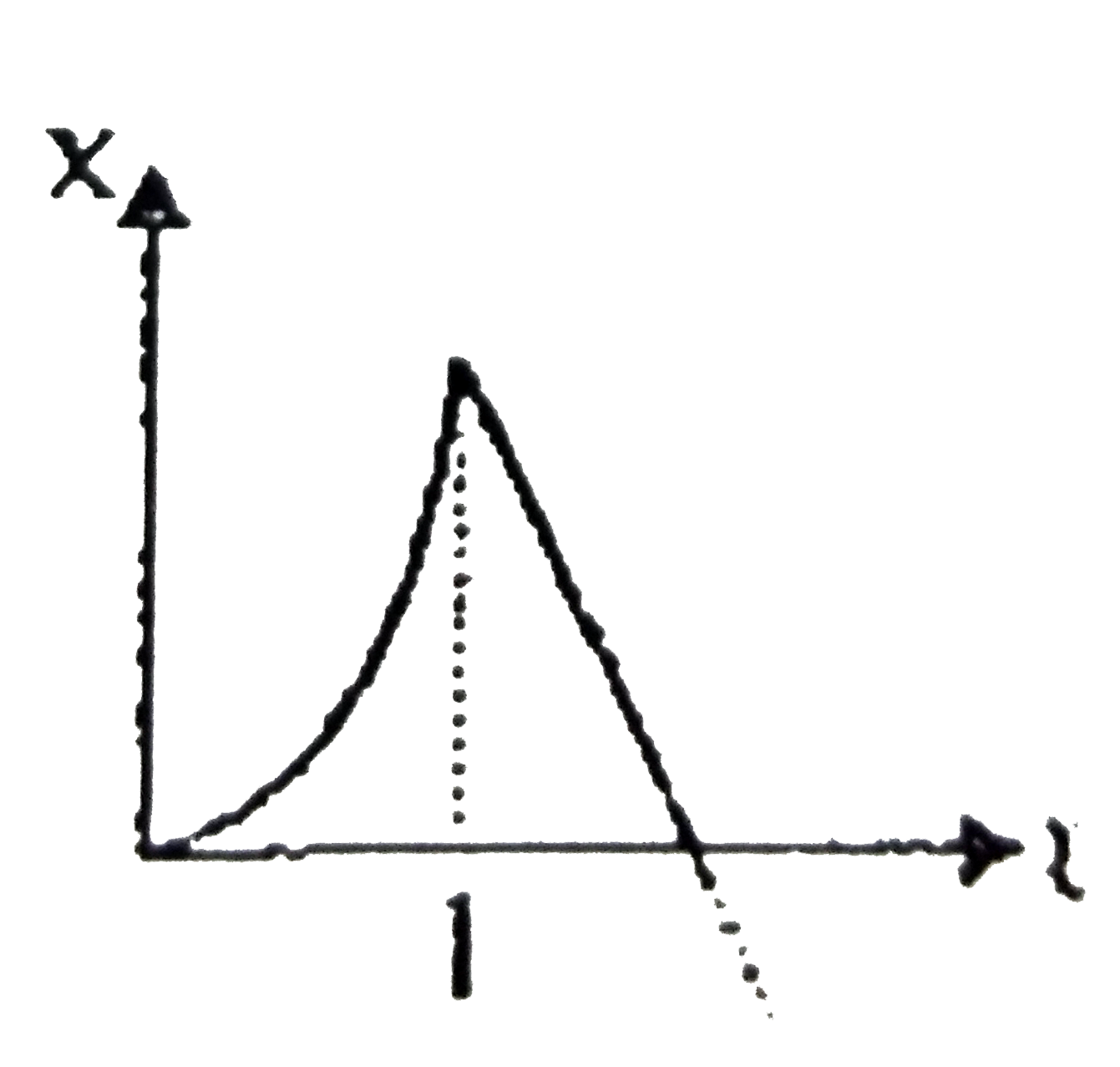
The velocity-time graph for a particle moving along X-axis is shown in the figure. The corresponding displacement-time graph is correctly shown by
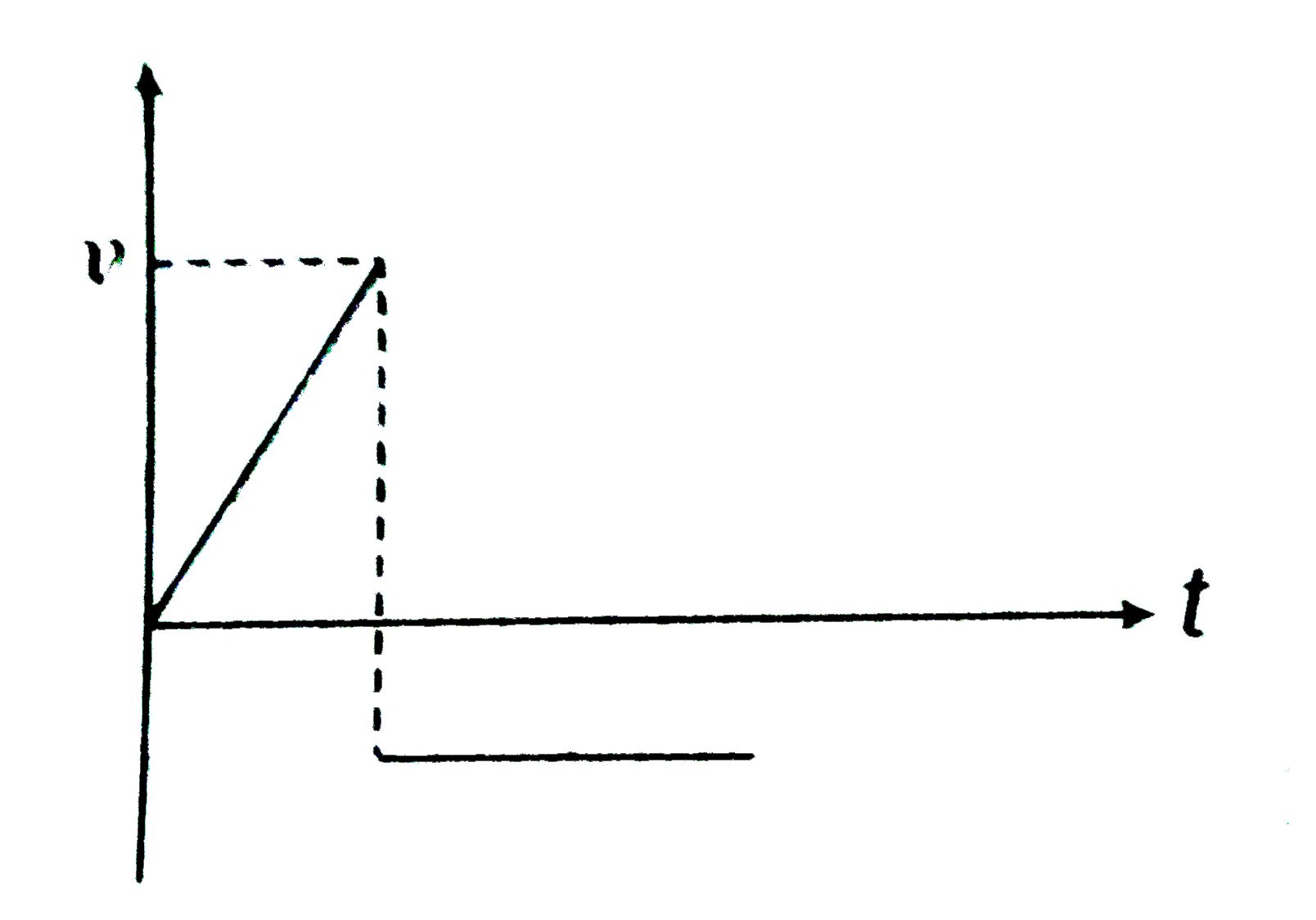
View SolutionAcceleration vs time graph is shown in the figure for a particle moving along a straight line. The particle is initially at rest. Find the time instant(s) when the particle comes to rest?
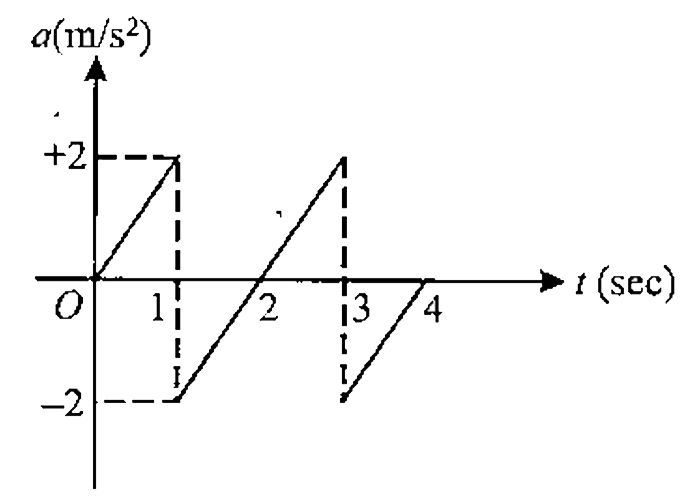
View SolutionAcceleration vs time graph is shown in the figure for a particle moving along a straight line. The particle is initially at rest. Find the time instant(s) when the particle comes to rest?

View SolutionPressure versus temperature graph of an ideal gas as shown in Fig.
Corresponding density (ρ) versus volume (V) graph will be
View SolutionThe graph of displacement vs time for a particle moving along a straight line is as shown. The best option for the corresponding velocity - time graph will be:
View Solution

