The fixed non-conducting cylinder shown in figure has a noconducting heavy piston of mass M that can slide without friction. The area of piston is S and the cylinder is filled with an ideal gas (λ=1.5) with an initial volume V and an initial pressure P. Assume that the outside pressure on the piston is zero (vaccum). (Neglect acceleration due to gravity).
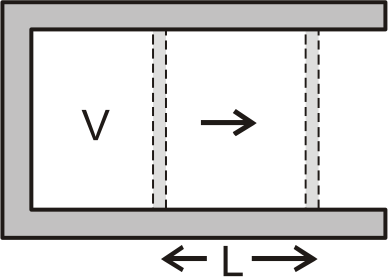
The initial acceleration of piston is:
PAλM
λPAM
PAM
PAλ−1
The initial force on the piston is PA
∵a=PAM
Similar Questions
एक सिलेण्डर जिसमे आयतन V तथा दाब P पर एक गैस भरी है, अनुप्रस्थ क्षेत्रफल A का एक पिस्टन लगा है | गैस बॉयल के नियम का पालन करती है | यदि पिस्टन को थोड़ा सा विस्थापित कर दिया जाए, तो कोणीय आकृति क्या है ?
View SolutionA piston divides a closed gas cylinder into two parts. Initially the piston is kept pressed such that one part has a pressure P and volume 5V and the other part has pressure 8P and volume V, the piston is now left free. Find the new pressure and volume for the isothermal and adiabatic process. (γ = 1.5).
View SolutionS is a piston of mass M which can slide inside a cylinder without friction. The walls of the cylinder is adiabatic and the piston is diathermic and area of cross section of cylinder is A. Length of the cylinder is 2l_0 . Initial pressure of each chamber is P_0 . Volume of the chambers are equal. Initially spring is unstretched and has spring constant k. The spring is fixed with piston and wall of the cylinder as shown in the figure. Find out its time period when piston is given small displacement.
View SolutionThe figure shows an insulated cylinder of volume V containing monoatomic gas in both the compartments. The piston is diathermic. Initially the piston is kept fixed, and the system is allowed to acquire a state of thermal equilibrium. If the initial pressures and temperatures are as shown.
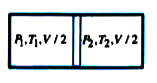
Calculate the final temperature and the final pressure. Now the pin which was keeping the piston fixed is removed and the piston set free to move. The piston is allowed to slide slowly, such that a state of mechanical equilibrium is also achieved (T1P2>P1T2)
View SolutionA piston divides a closed gas cylinder into two parts. Initially the piston is kept pressed such that one part has a pressure P and volume 5Vand the other part has pressure 8P and volume V, the piston is now left free. Find the new pressure and volume for the isothermal and adiabatic process.
View SolutionA piston divides a closed gas cylinder into two parts. Initially the piston is kept pressed such that one part has a pressure P and volume 5V and the other part has pressure 8P and volume V, the piston is now left free. Find the new pressure and volume for the isothermal and adiabatic process. (γ=1.5).
View SolutionThe pressure of a gas contained in a cylinder with a movable piston is 300 Pa. The area of the piston is 0.5 m2. Calculate the force that is exerted on the piston.
View SolutionThe fixed non-conducting cylinder shown in figure has a noconducting heavy piston of mass M that can slide without friction. The area of piston is S and the cylinder is filled with an ideal gas (lambda=1.5) with an initial volume V and an initial pressure P . Assume that the outside pressure on the piston is zero (vaccum). (Neglect acceleration due to gravity). The initial acceleration of piston is:
View Solution

