a.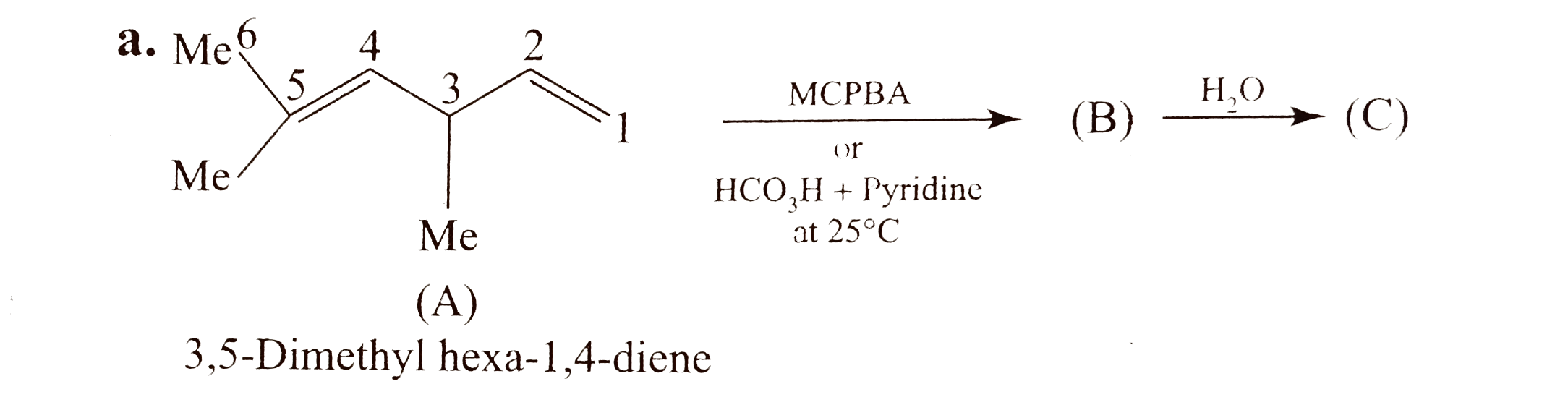
b. 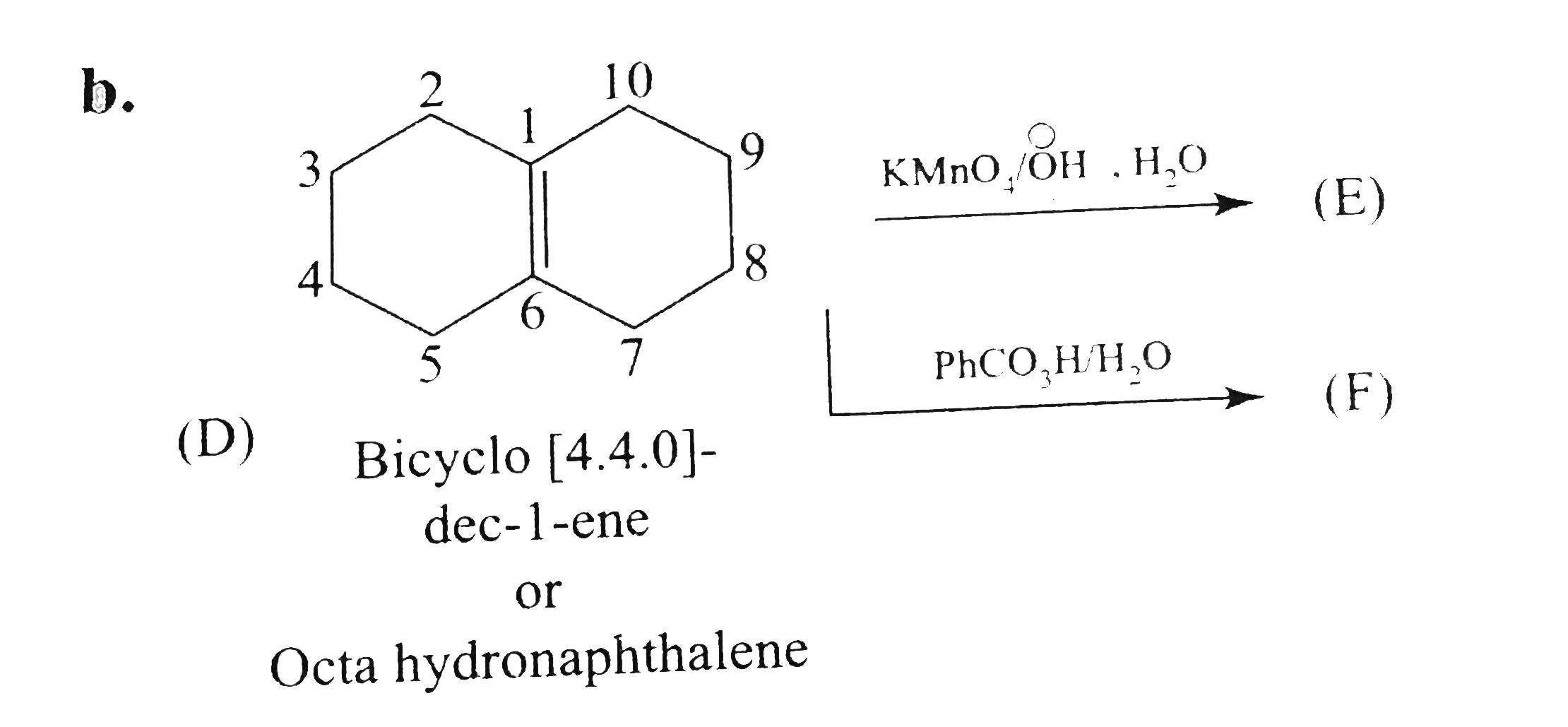
c. 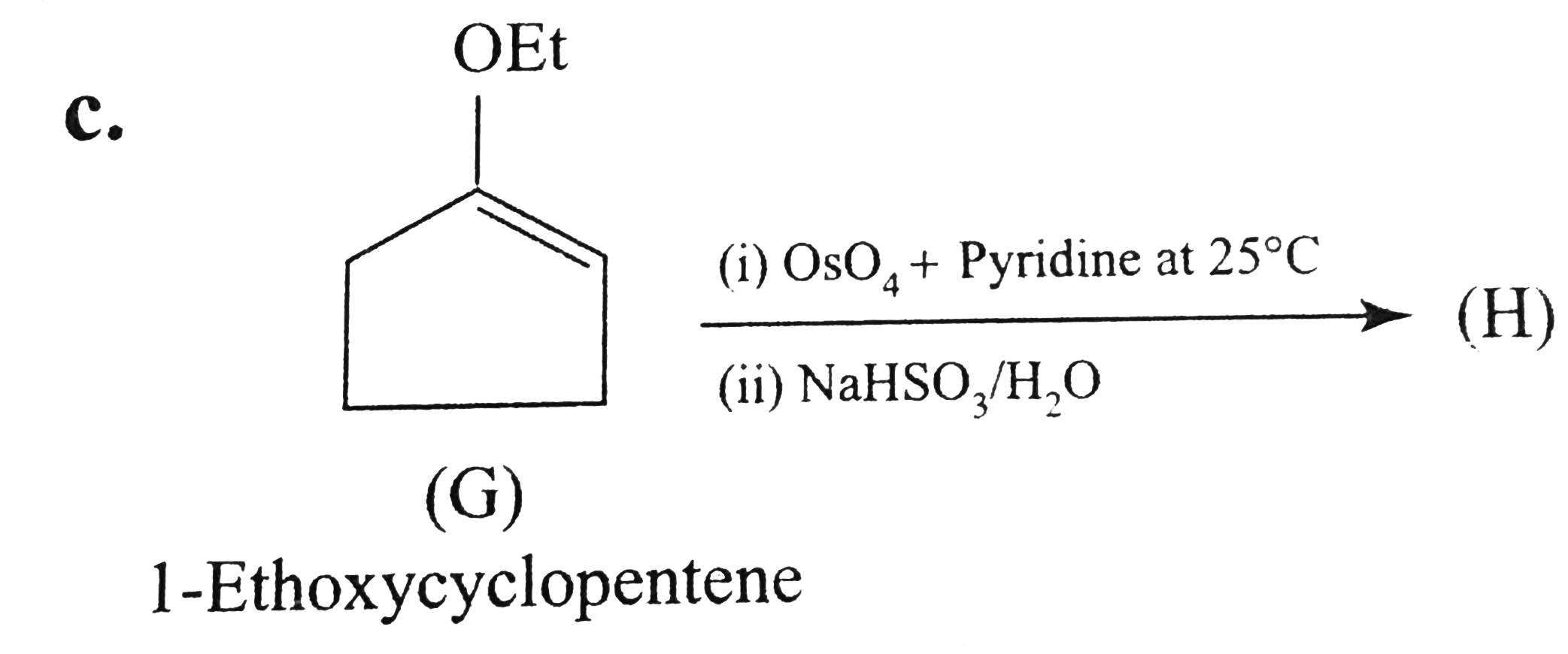
d. 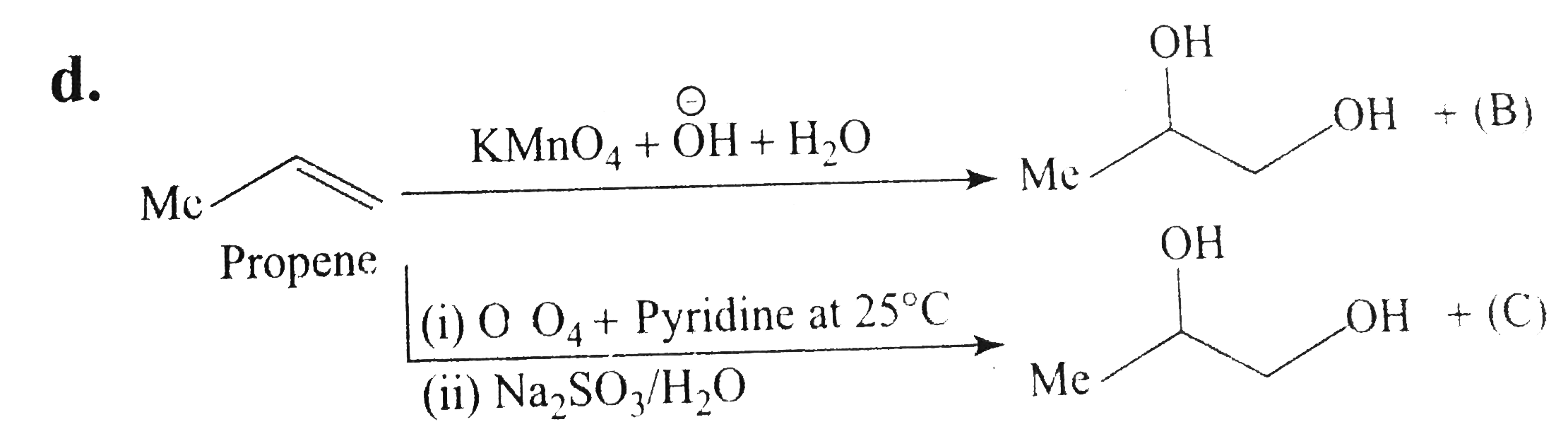
What is the oxidation number of Mn in (B) and oxidation number of Os in (C) ?
Video Solution
Text Solution
a. 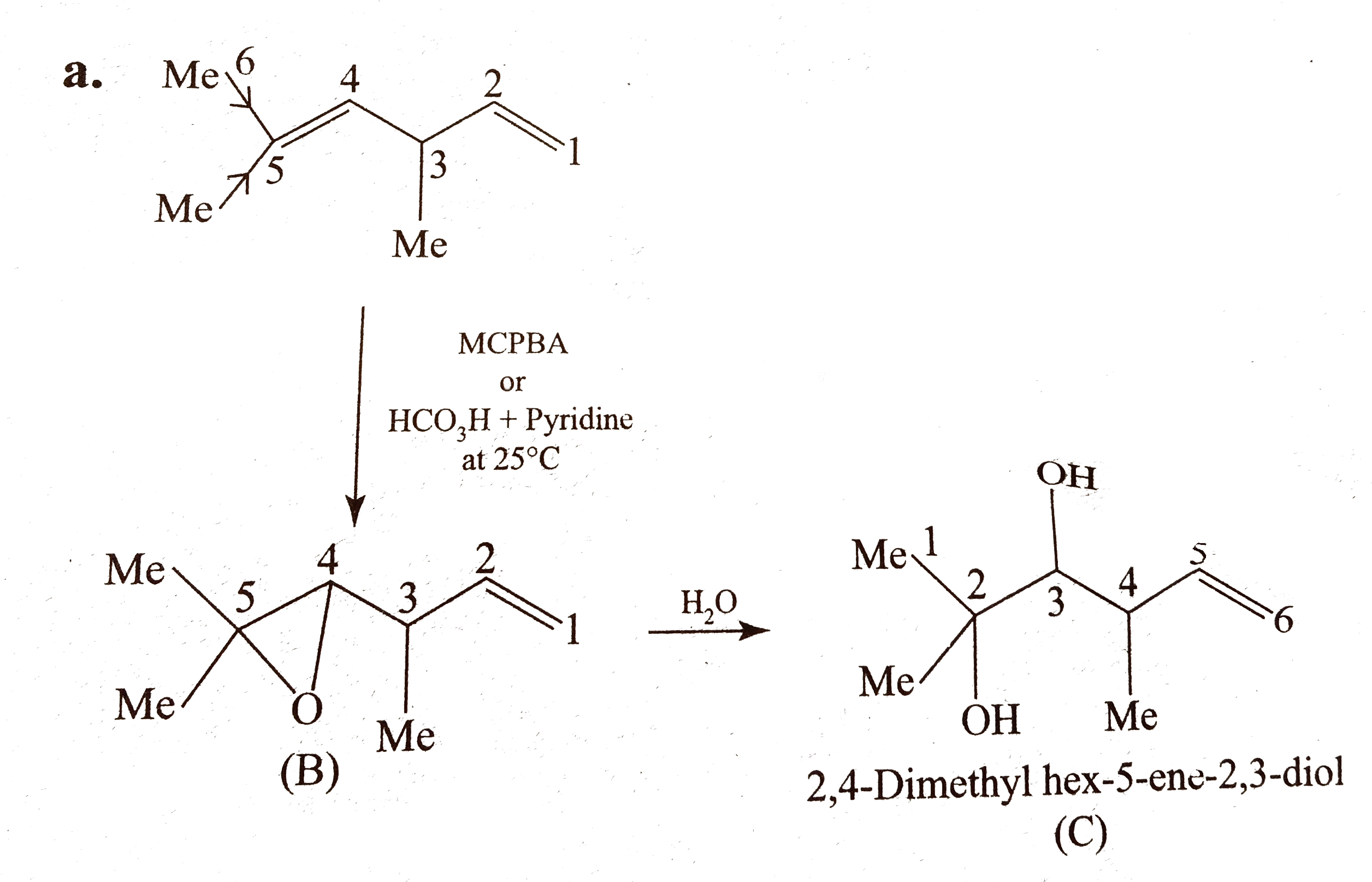
b. 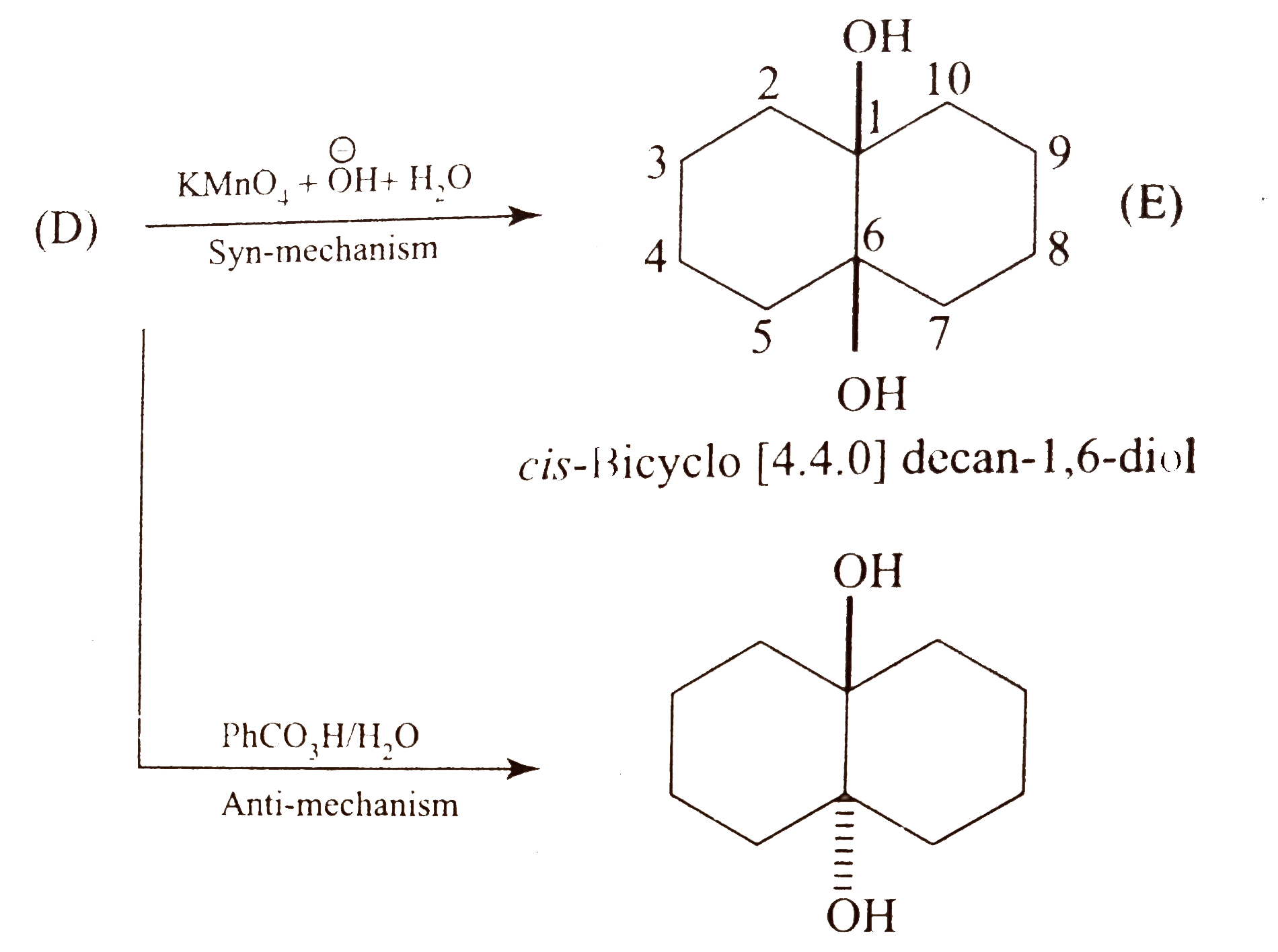
c. 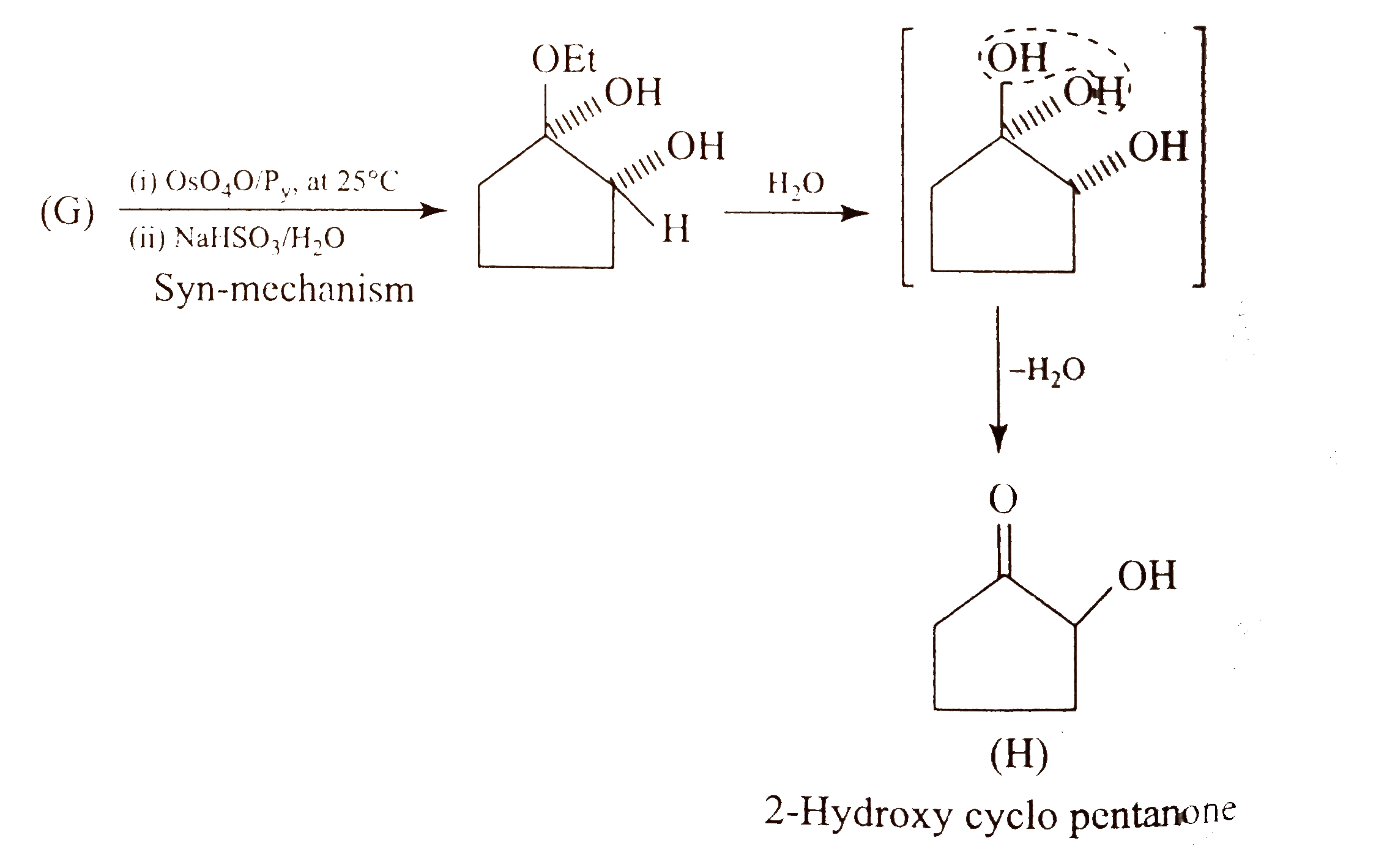
d. (B)⇒MnO2,(C)⇒Os
7+MnO4→4+MnO4(B)
Oxidation state of Mn in MnO2=+4
8+OsO4→Os
Oxidation state of Os is zero.
|
Similar Questions
Knowledge Check
the oxidation number of C in CO2 is
A−2B+2C−4D+4The oxidation number of Mn is +7 in
Amanganese dioxideBmanganese chlorideCmanganese sulphateDpotassium permanganateThe oxidation number of Mn is +2 in
Amanganese oxideBmanganese chlorideCmanganese sulphateDpotassium permanganate

