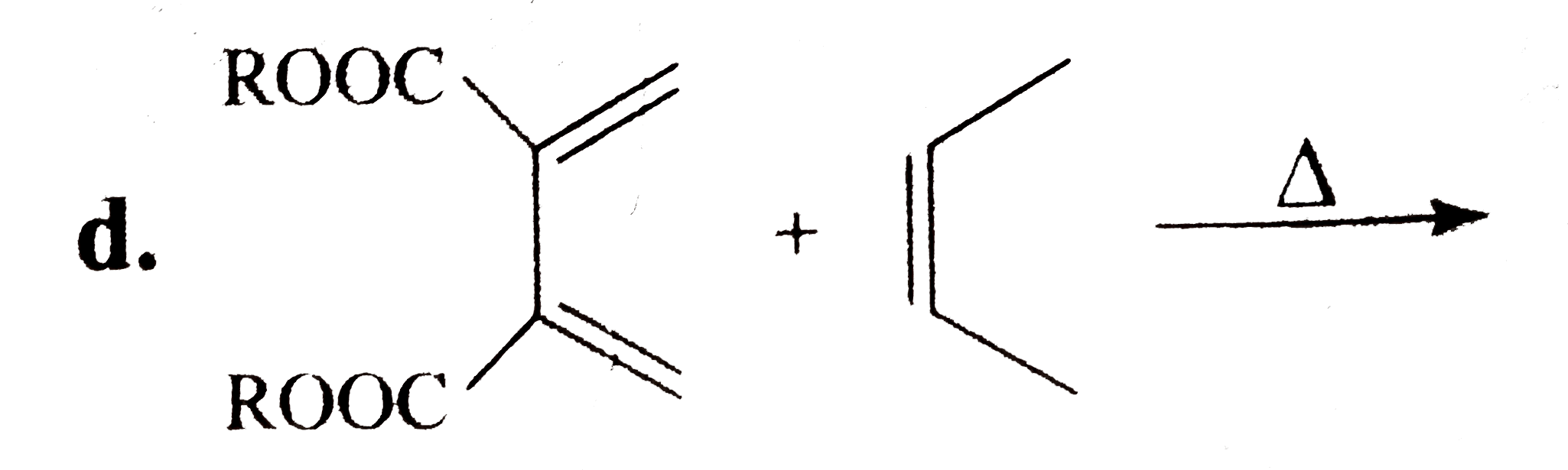
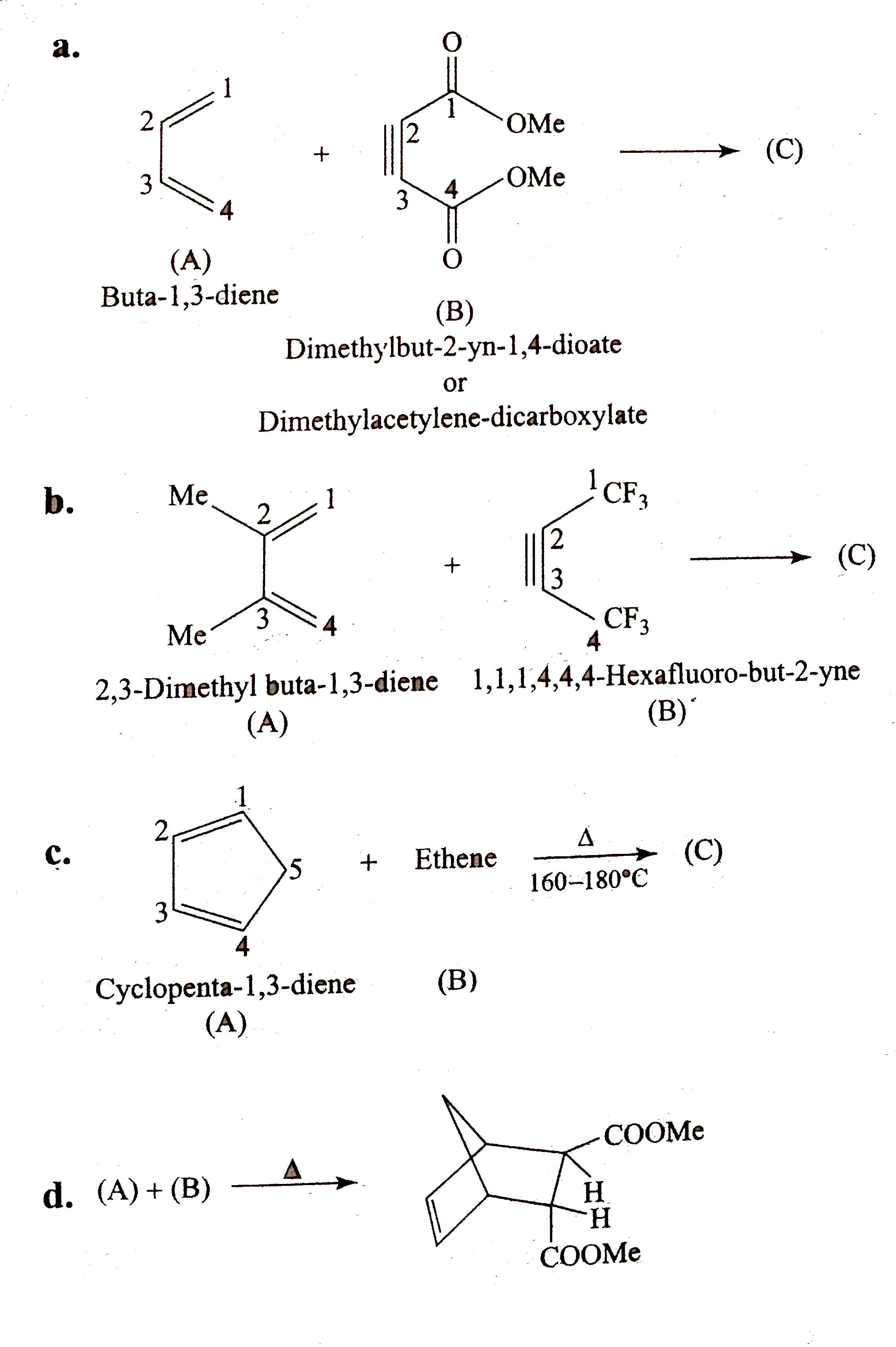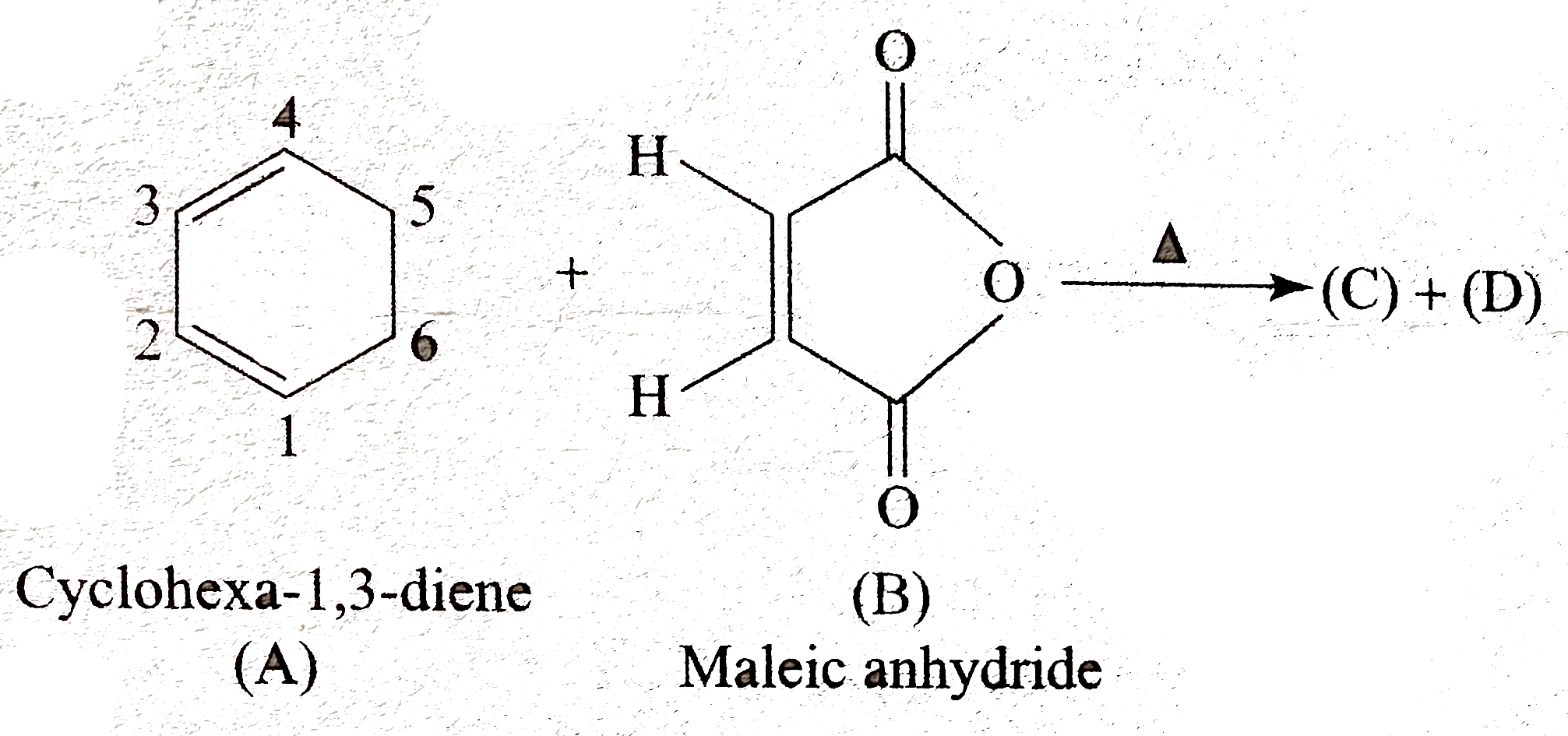Which of the following Diels− Alder reactions do not take place ?
A
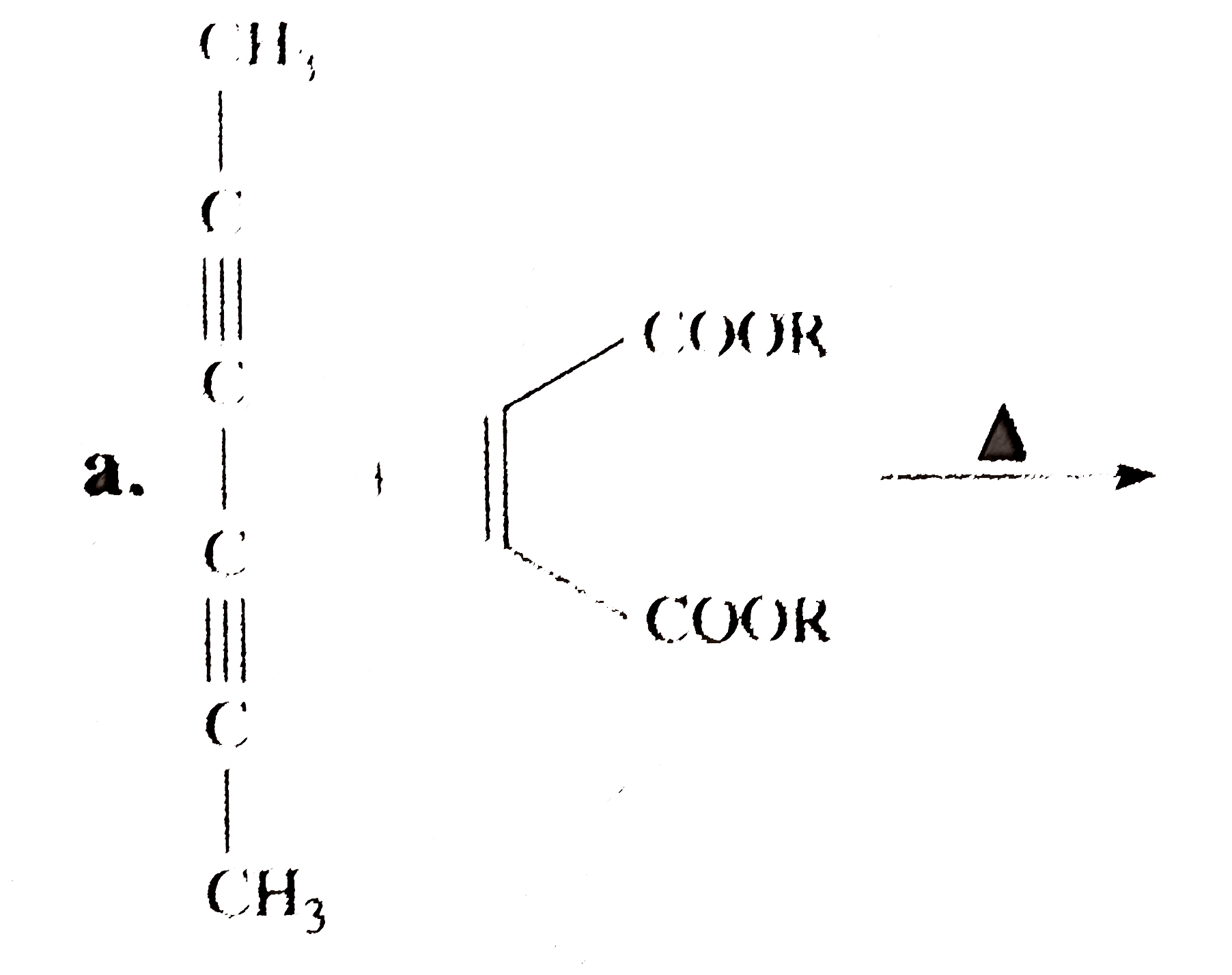
B
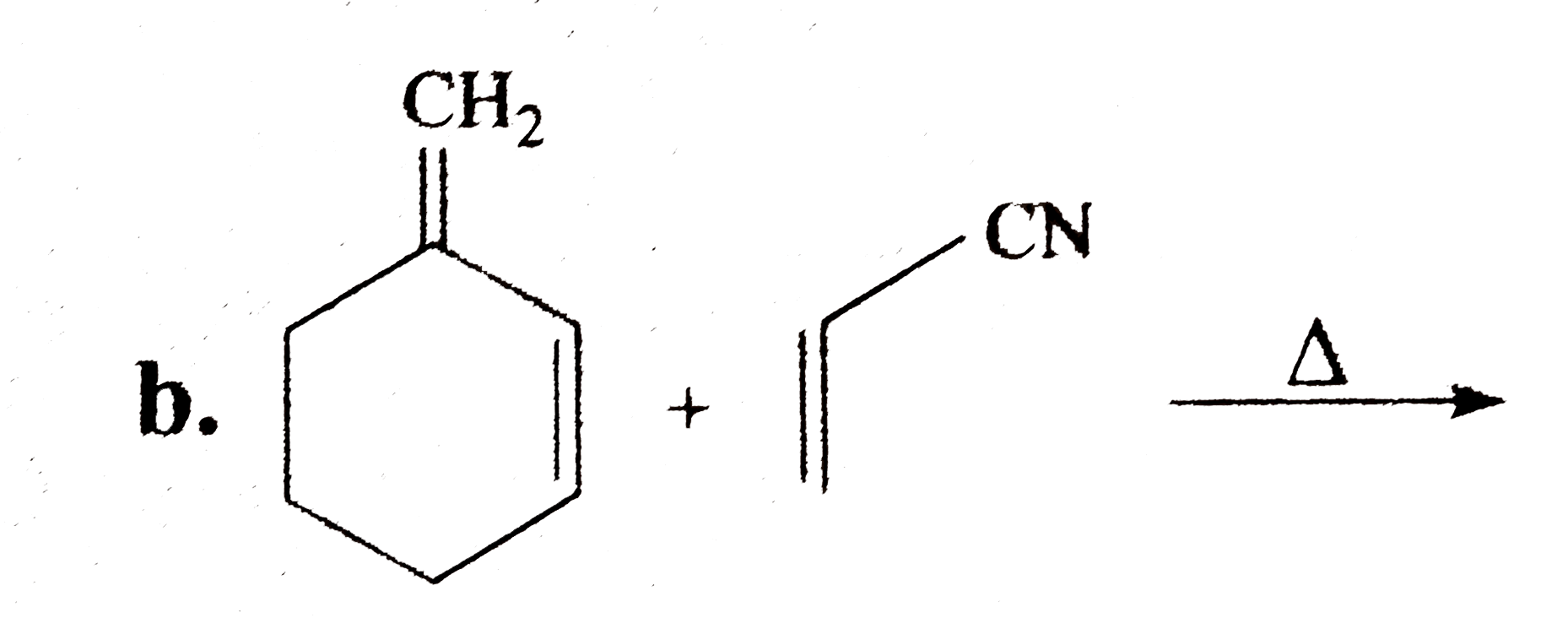
C

D
Video Solution
Text Solution
The correct Answer is:a,b,c,d
Diels − Alder reaction takes place in the following cases :
1. Dienes with EDG.
2. Dienophile with EWG.
3. Dienes should have S−cis conformation and should be non− linear.
(a) is not possible because diyne is linear.
(b) and (c) are not possible becasue in both the cases dienes have S−trans conformation.
(d) is not possible because dienes have EWG and dienophiles have EDG(it should be reverse).
|
Similar Questions
Knowledge Check
Which of the following reactions does not take place in the smelting process?
AFeO+CO→Fe+CO2BCaO+SiO2→CaSiO3CC+CO2→2CODFe2O3.H2O→Fe2O3+H2OWhich of the following reactions does not take place during rusting ?
AH2CO3⇔2H++CO2−3B4Fe2++O2(dry)→Fe2O3C4Fe2++O2+4H2O→2Fe2O3+8H+DFe2O3+xH2O→Fe2O3.xH2OWhich of the following reactions does not take place during rusting?
AH2CO3⇔2H++CO2−3B4Fe2++O2(dry)→Fe2O3C4Fe2++O2+4H2O→2Fe2O3+8H+DFe2O3+xH2O→Fe2O3.xH2O
Haloform reaction does not take place with
View SolutionWhich out of the following reactions does not take place ?
View SolutionWhich of the following reaction does not take place at cathode
View SolutionHaloform reaction does not take place with :
View SolutionIonic reactions takes place in
View Solution