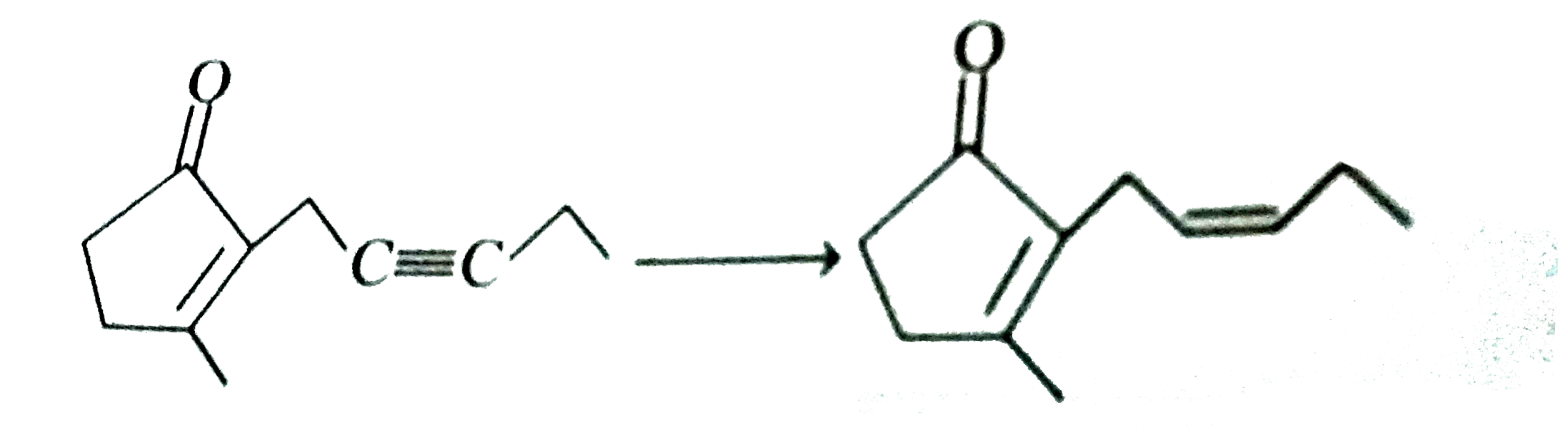
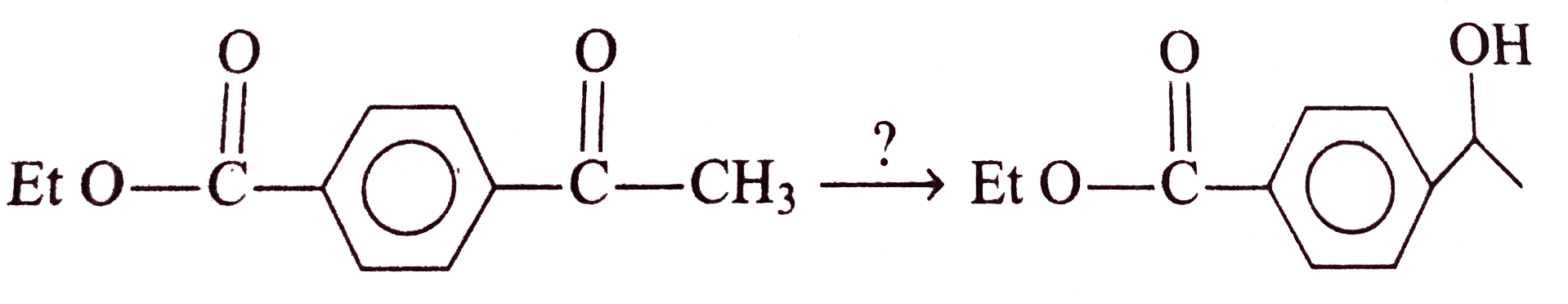Consider the following reactions:

Which of the following group(s) of reagents is/are used in the above conversion?
A
a.(A)⇒AcidicKMnO4,(B)⇒Ca(OH)2,
(C)⇒Zn−Hg/HCl
B
b.(A)⇒O3/Ph3P,(B)⇒Ba(OH)2,(C)⇒LAH/ether
C
c.(A)⇒O3/H2O,(B)⇒Sr(OH)2,
(C)⇒NH2NH2/ɵOH
D
d.(A)⇒O3/Ag2O,(B)⇒Ba(OH)2,(C)⇒HI+RedP
Video Solution
Text Solution
The correct Answer is:A, C, D
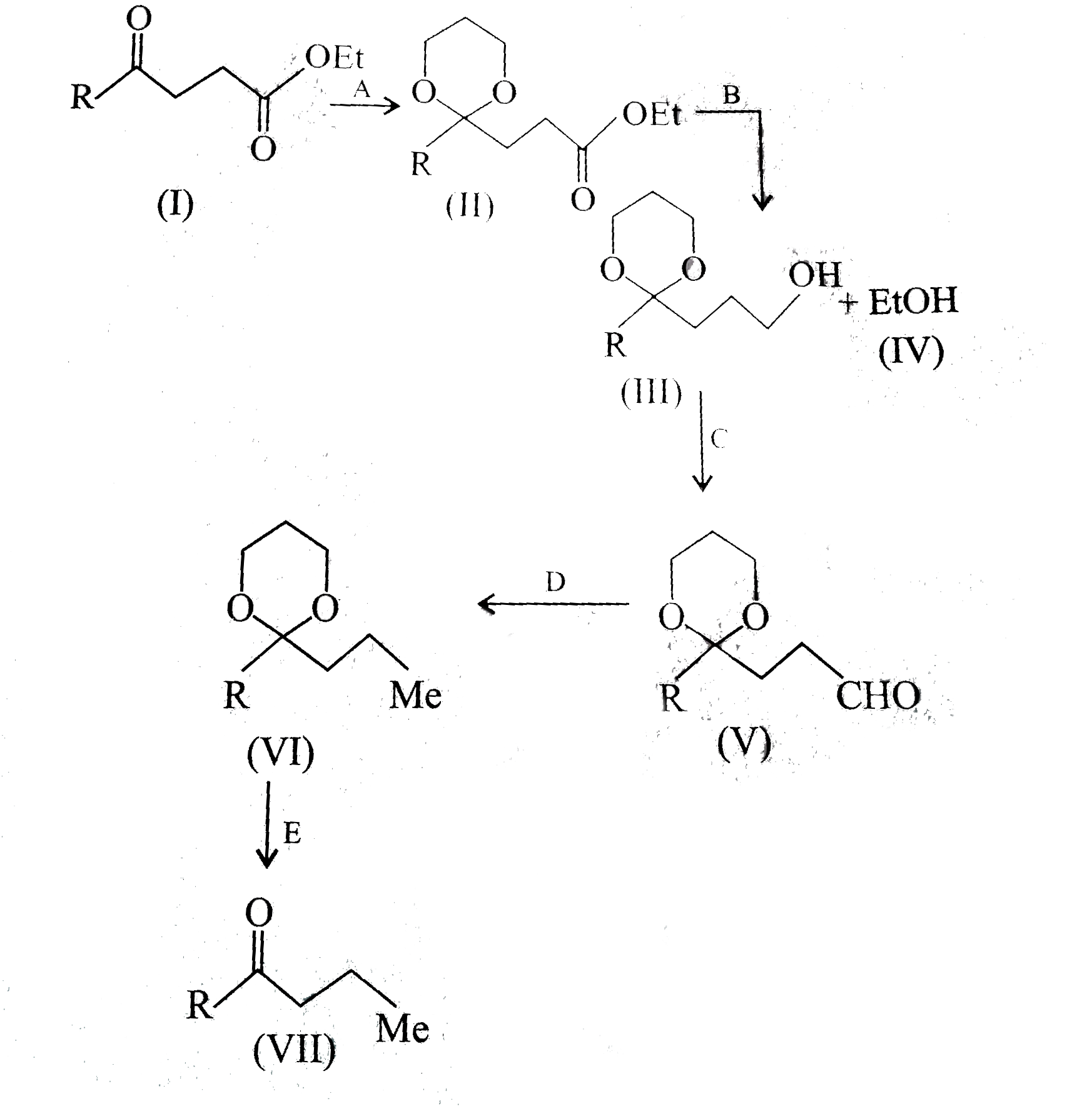
A ⇒ The oxidation of (C=C) to acids can be carried out by either of the following: (i) acidic KMnO4, (ii) ozonolysis following by hydrolysis (O3/H2O), (iii) ozonolysis followed by oxidation with Ag2O,H2O2, peracid, or KMnO4/NaIO4
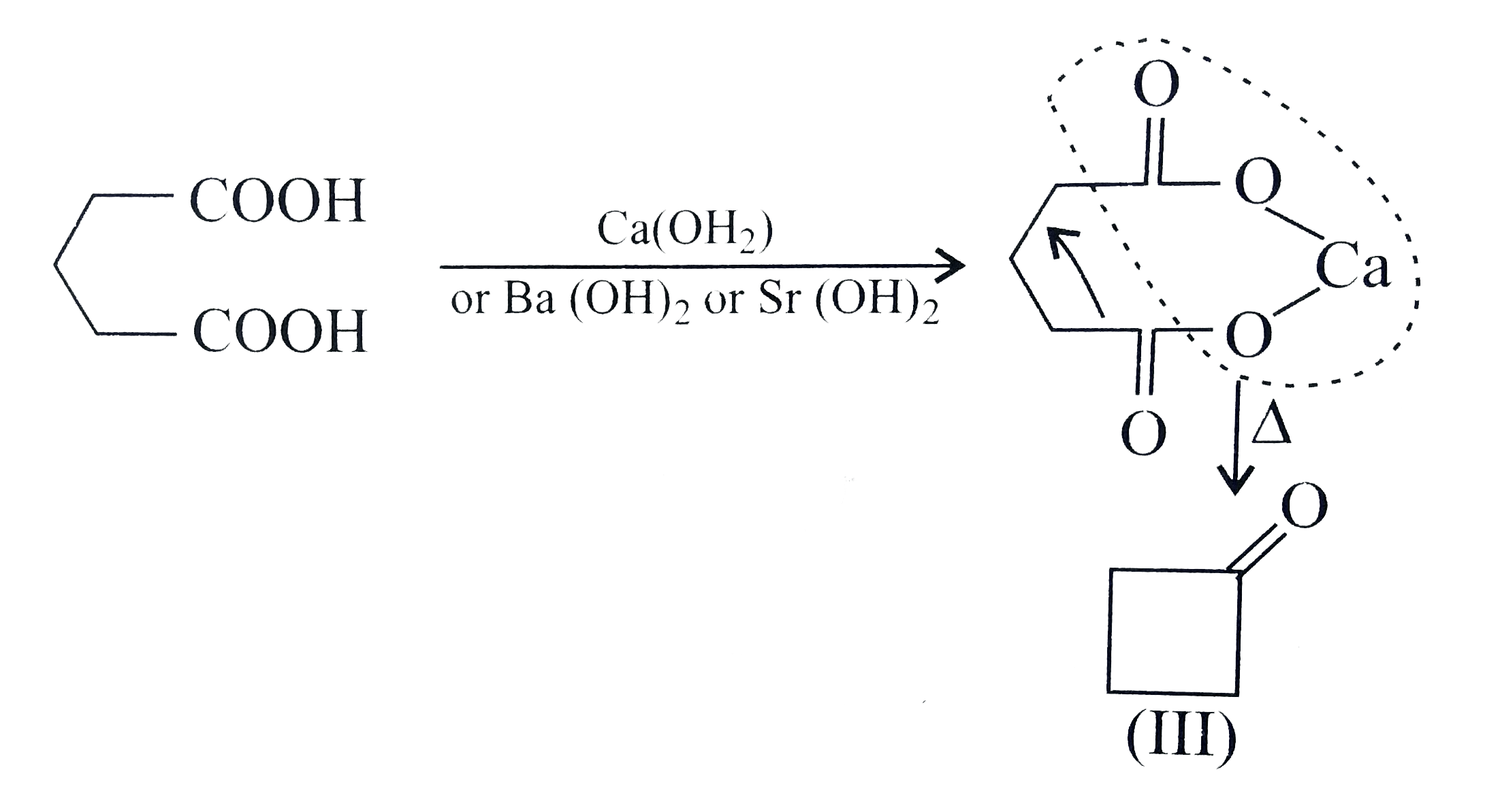
B ⇒ (II) is converted to Ca, Ba, or Sr salt by reacting with their hydroxides which on dry distillation or heating gives cyclic ketone with one C atom less than in II.
C⇒ The
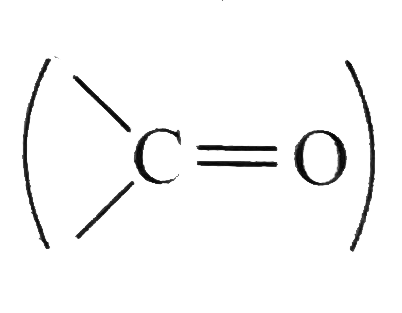
group can be converted to (−CH2−) by either of the following:
i. Clemmensen's reduction (Zn−Hg/HCl),
ii. Wolff-Kishner reduction (NH2NH2+ɵOH), or
iii. HI+P
So the answer are (a), (c), and (d).
|
Similar Questions
Knowledge Check
Consider the following reactions:
Which of the following reagents is/are used in the above conversion?A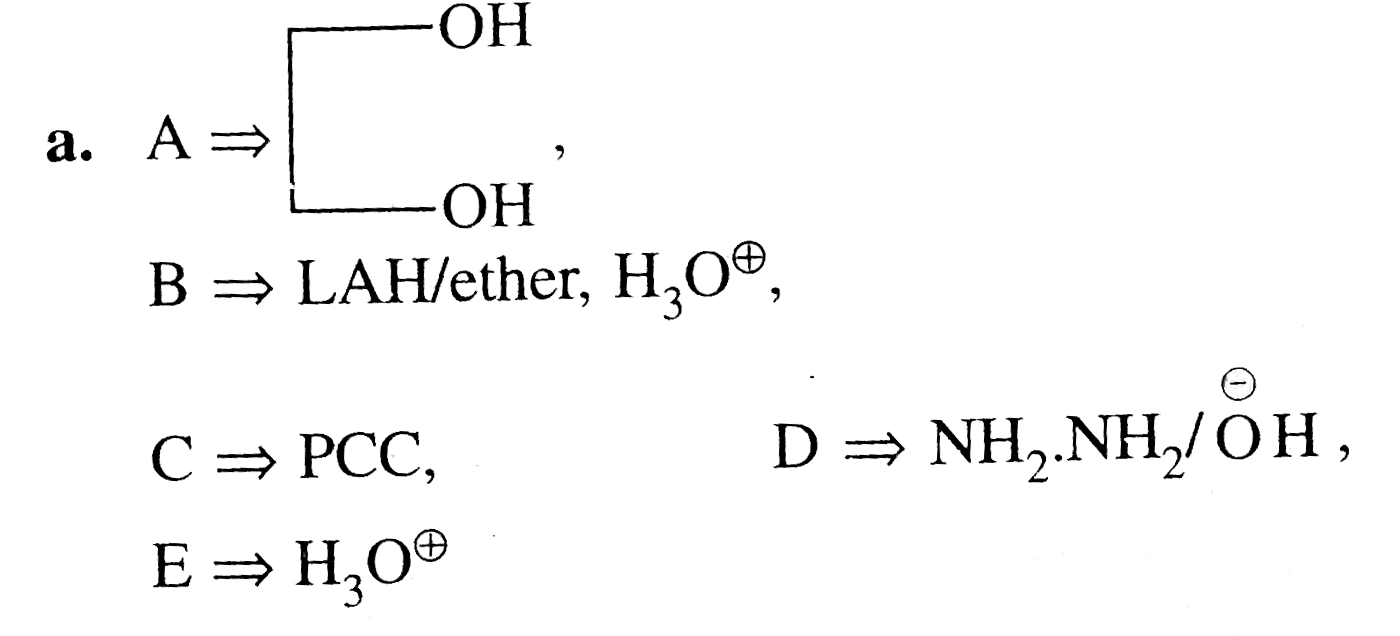 B
B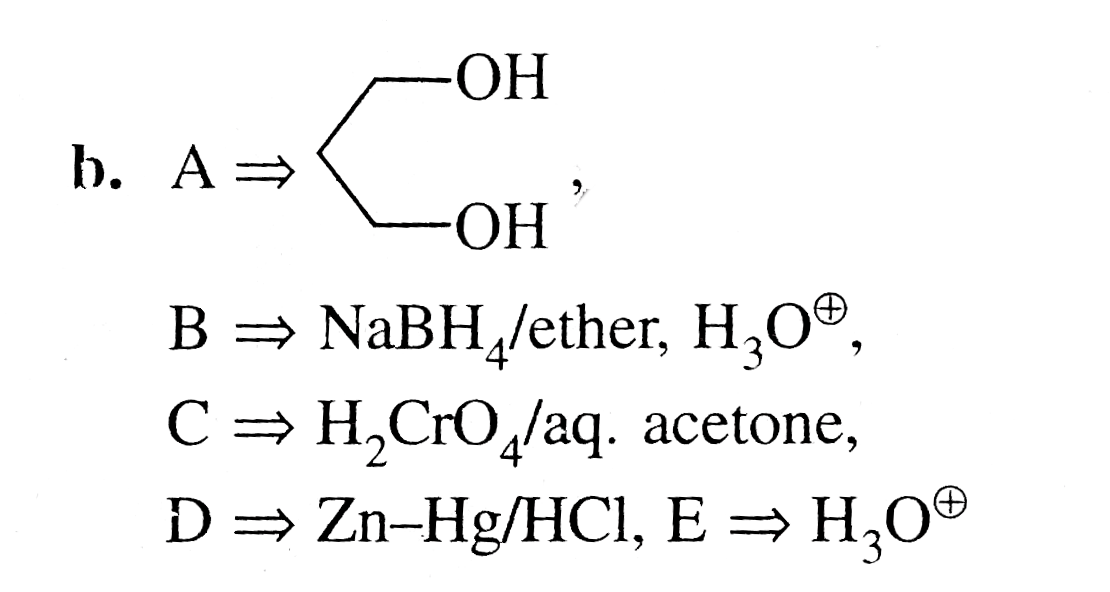 C
C D
D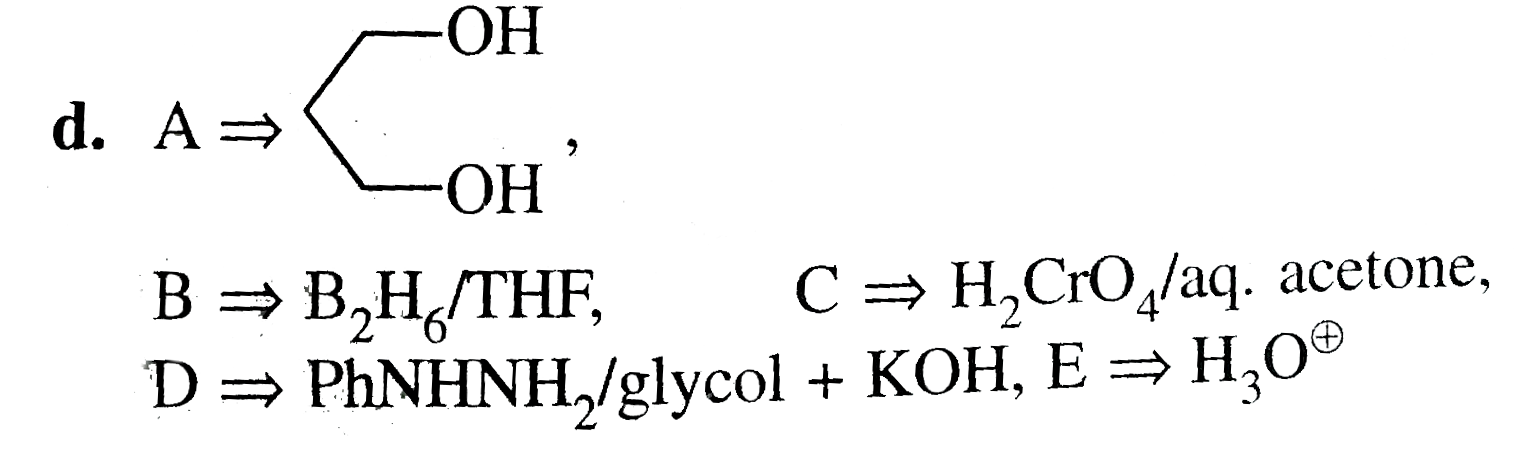
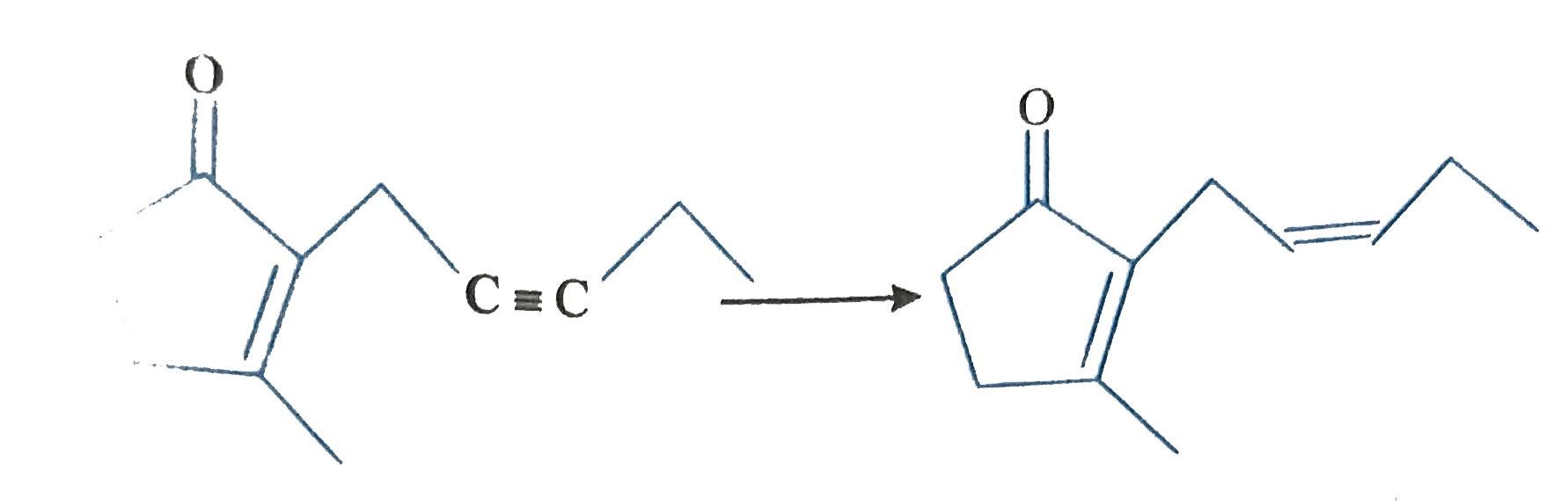
Which reagent will be used for the above conversion?ANa/Liq.NH3BH2,Pd−CaCO3CLi,Ph−NH2DH2,Pt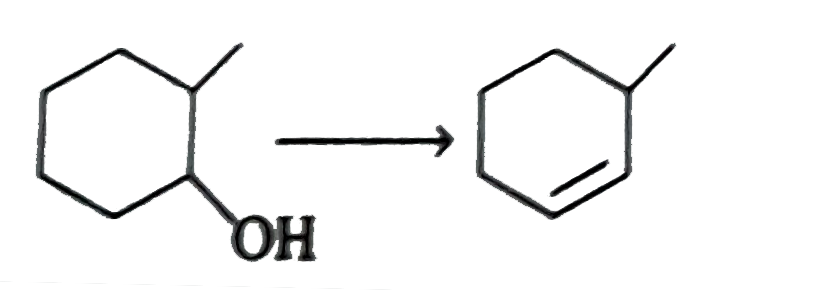
Which of the following is best set of reagents to performs to the above conversion?AThO2,ΔBH3PO4,ΔCConc.H2SO4,ΔDAl2O3,Δ

Which of the following is best set of reagents to performs to the above conversion?
View Solution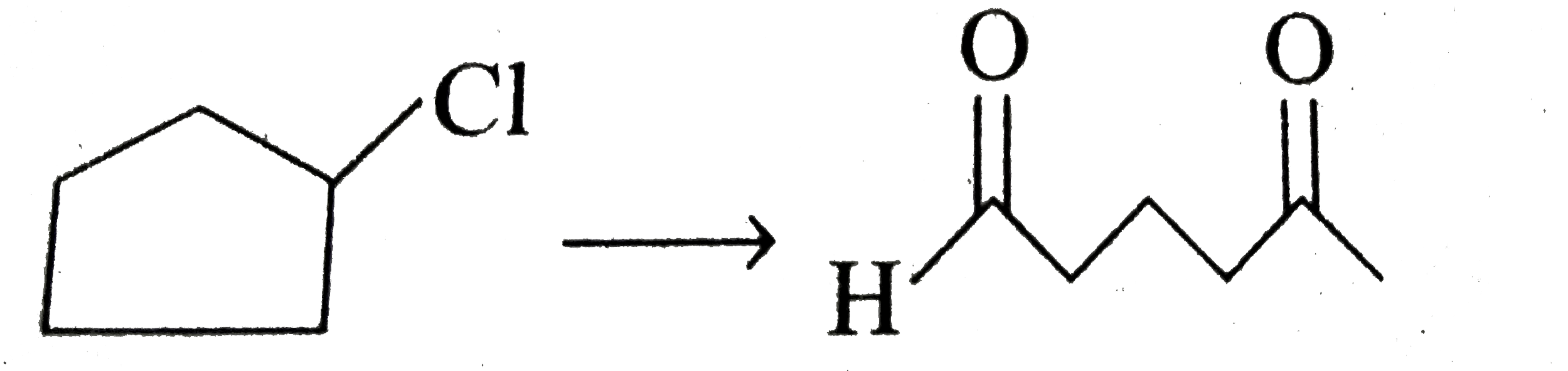
Which of the following is the correct option of reagent for the above conversion ?
View SolutionWhich of the following reagents are used in the conversion of
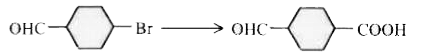
View SolutionIn the given reaction,
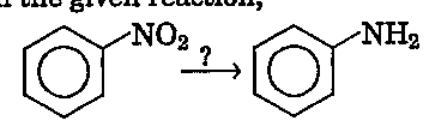
Which of the following reagent (s) is/are used in the given reaction?
View Solution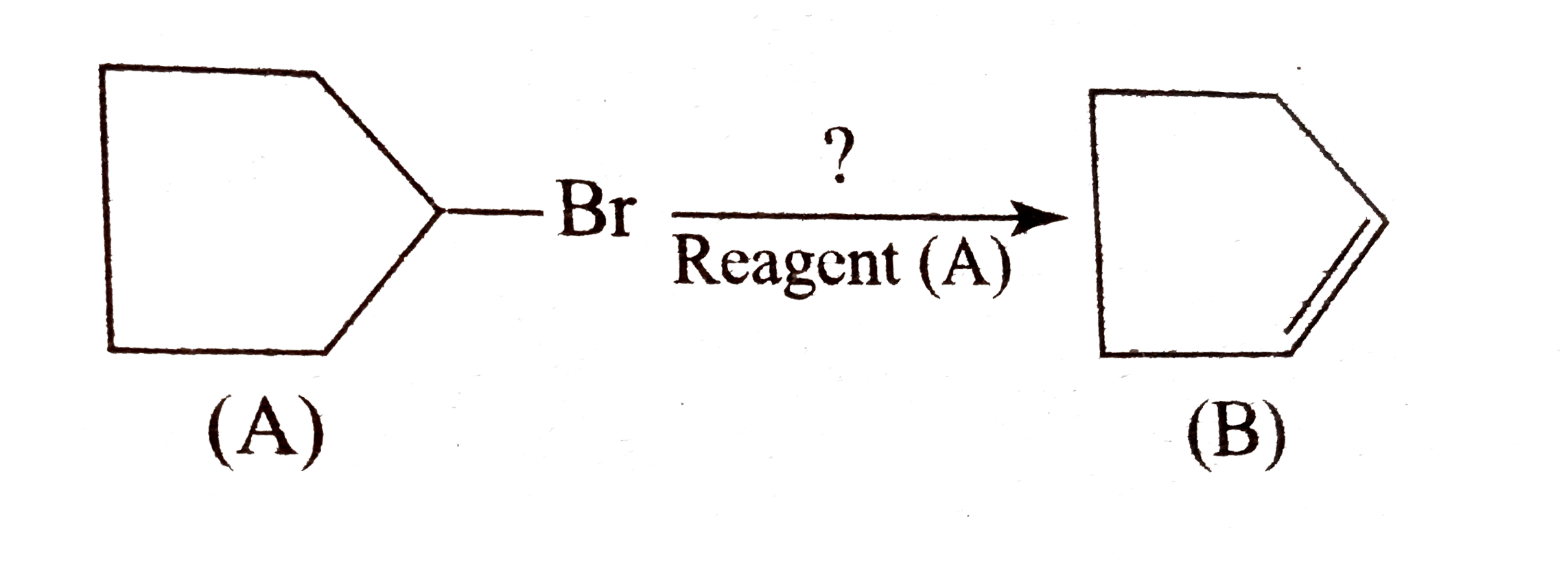
Which reagent cannot be used for the above conversion ?
View Solution