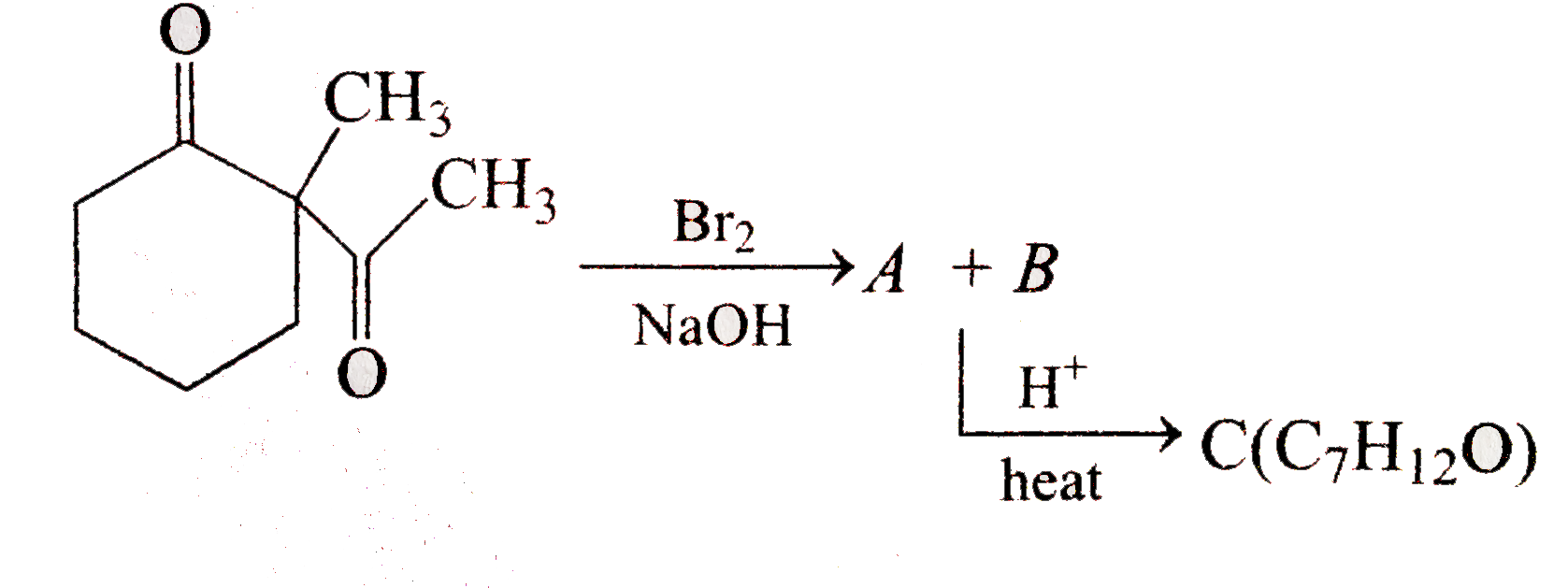
Identify (A),(B), and (C) and give their strucyures.
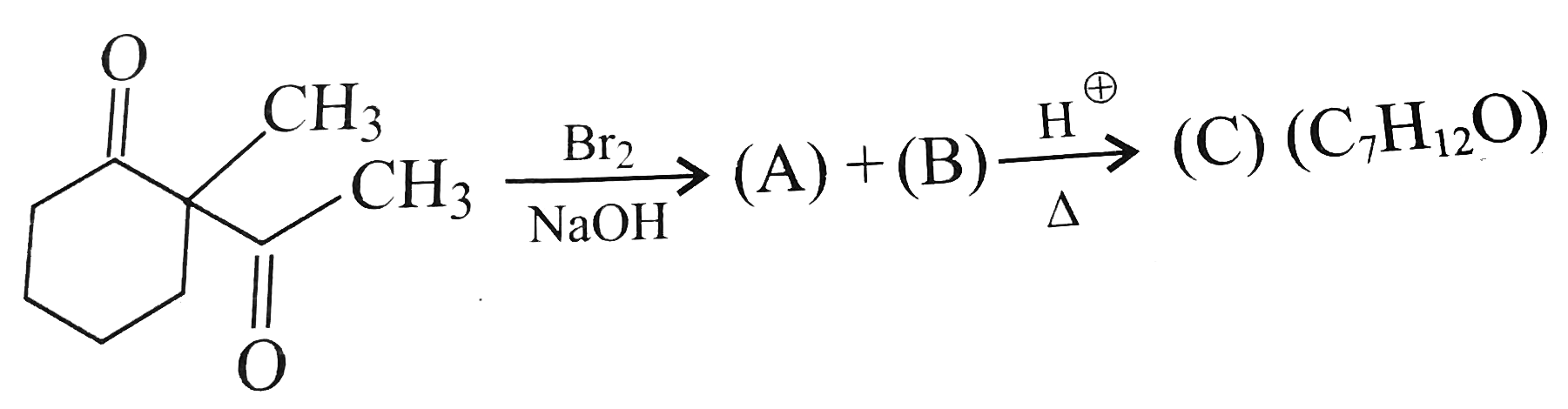
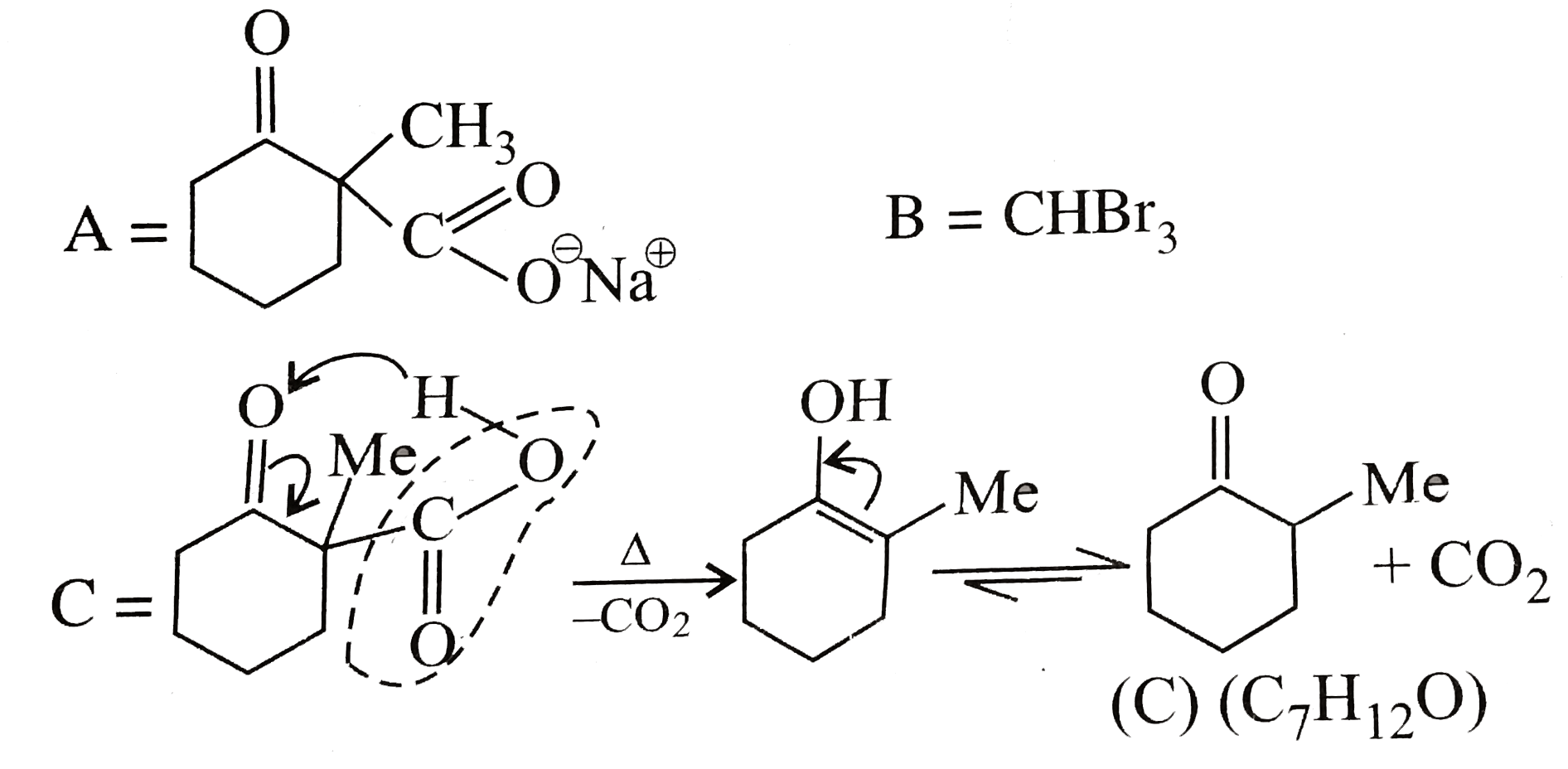
β-Keto acid undergoes decrdoxylation (neighbouring group participation).
Topper's Solved these Questions
- REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSBook:CENGAGE CHEMISTRYChapter:REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSExercise:SUBJECTIVE TYPE
Explore 3 Videos
- REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSBook:CENGAGE CHEMISTRYChapter:REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSExercise:Exercise (Assertion And Reasoning)
Explore 10 Videos
- QUALITATIVE INORGANIC SALT ANALYSISBook:CENGAGE CHEMISTRYChapter:QUALITATIVE INORGANIC SALT ANALYSISExercise:Viva Voce Questions And Part-C (Analysis Of Cations)
Explore 42 Videos
- SOLID STATEBook:CENGAGE CHEMISTRYChapter:SOLID STATEExercise:Ex 1.2 (Objective)
Explore 9 Videos
Similar Questions
Knowledge Check
Identify A,B,C and D:
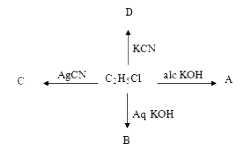 AA=C2H4,B=C2H5OH,C=C2H5NC,D=C2H5CNBA=C2H5OH,B=C2H4,C=C2H5CN,D=C2H5NCCA=C2H4,B=C2H5OH,C=C2H5CN,D=C2H5NCDA=C2H5OH,B=C2H4,C=C2H5NC,D=C2H5CN
AA=C2H4,B=C2H5OH,C=C2H5NC,D=C2H5CNBA=C2H5OH,B=C2H4,C=C2H5CN,D=C2H5NCCA=C2H4,B=C2H5OH,C=C2H5CN,D=C2H5NCDA=C2H5OH,B=C2H4,C=C2H5NC,D=C2H5CN
An alkali metal A gives a compound B (molecular mass = 40) on reacting with water. The compound B gives a soluble compound C on treatment with aluminium oxide. Identify A, B and C and give the reactions involved.
View SolutionAn alkali metal A gives a compound B (molecular mass = 40) on reacting with water. The compound B gives a soluble compound C on treatment with aluminium oxide. Identify A, B and C and give the reactions involved.
View SolutionAn alkali metal 'A' gives a compound B (molecular mass = 56) on reacting with water. The compound 'B' gives a soluble compound 'C' on treatment with aluminium oxide. Identify A, B and C and give the reactions involved.
View SolutionAn element A reacts with water to form a compound B which is used in white washing. The compound B on heating forms an oxide C which on treatment with water gives back B. Identify A, B and C and give the reactions involved.
View SolutionAn element A reacts with water to form a compound B which is used in white washing. The compound B on heating forms an oxide C which on treatment with water gives back B. Identify A, B and C and give the reactions involved.
View SolutionAn element A reacts with water to form a compound B which is used in white washing. The compound B on heating forms an oxide C which on treatment with water gives back B. Identify A, B and C and give the reactions involved.
View SolutionIdentify [A][B] and [C] and gives the complete chemical reactions involved.
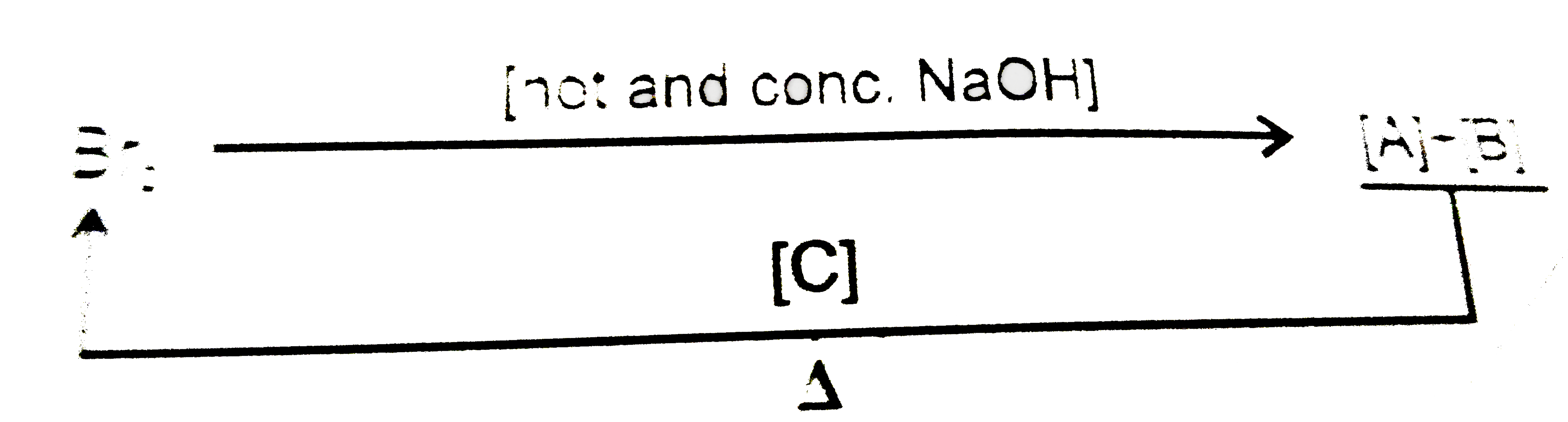
View Solution